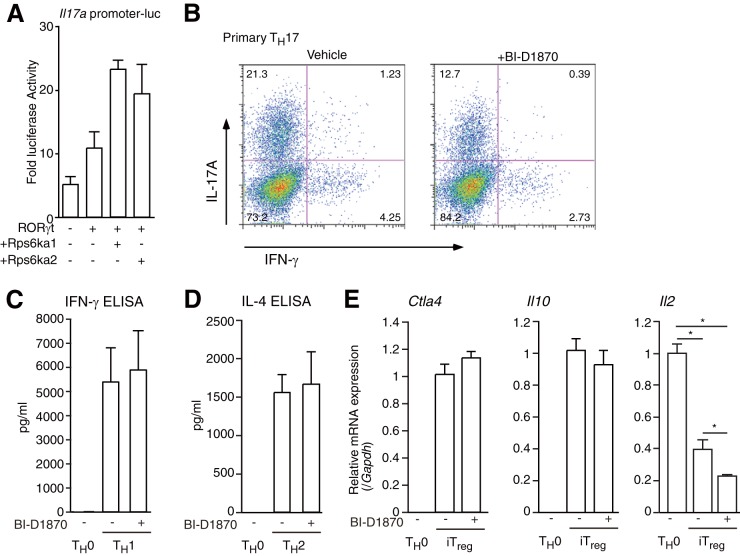
FIG 7.
Effect of BI-D1870 on TH17, TH1, TH2, and iTreg differentiation. (A) Luciferase reporter assays of the mIl17a promoter in 68-41 cells. After transfection with a β-gal expression vector, an mIl17a promoter luciferase vector, and/or an expression vector for RORγt, Rps6ka1, or Rps6ka2, 68-41 cells were cultured for 36 h and collected and luciferase assays were performed. As an internal control, ONPG-dependent β-gal activities were measured with a microplate reader. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (B) Flow cytometric analysis of primary cultured CD4+ T cells under TH17 conditions (anti-CD3ε, -CD28, -IFN-γ, and -IL-4 antibodies; 10 ng ml−1 IL-6; and 1 ng ml−1 TGF-β) with or without 10 μM BI-D1870 for 4 days. The cells were collected, and flow cytometric analysis was performed. (C) ELISA of IFN-γ protein in primary cultured TH0 cells and TH1 cells treated with or without BI-D1870. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (D) ELISA of IL-4 protein in primary cultured TH0 cells and TH2 cells treated with or without BI-D1870. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05. (E) Real-time RT-PCR analysis of iTreg cells treated with or without 10 μM BI-D1870. After the isolation of RNAs from each tissue type, RT-qPCRs were performed. The mRNA levels of all genes were normalized to the level of Gapdh mRNA expression. Each experiment was performed at least three times, and results are presented as means ± SD. *, P < 0.05.