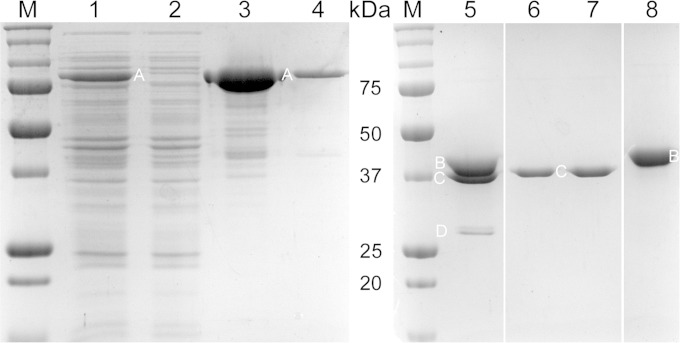
FIG 7.
Purification of B. anthracis HtrC. A His6-MBP-HtrC45–391 fusion protein was overexpressed in E. coli, purified by metal affinity chromatography, cleaved with TEV protease, and separated from the His6-MBP tag and His6-TEV using a second metal affinity column. Samples were soluble protein extracts of induced (lane 1) and uninduced (lane 2) cells, eluate fractions from the metal affinity column containing His6-MBP-HtrC45–391 (A) (lanes 3 and 4), His6-MBP-HtrC45–391 digested with His6-TEV protease (D) (lane 5), flowthrough fractions from the second metal affinity column containing HtrC45–391 (C) (lanes 6 and 7), and eluate from the second metal affinity column containing His6-MBP (B) (lane 8). Lanes 1 to 4 and 5 to 8 are from two different gels; the intervening lanes between lanes 5 and 6 and lanes 7 and 8 were removed for clarity. The masses of standard proteins (M) are indicated in the center.