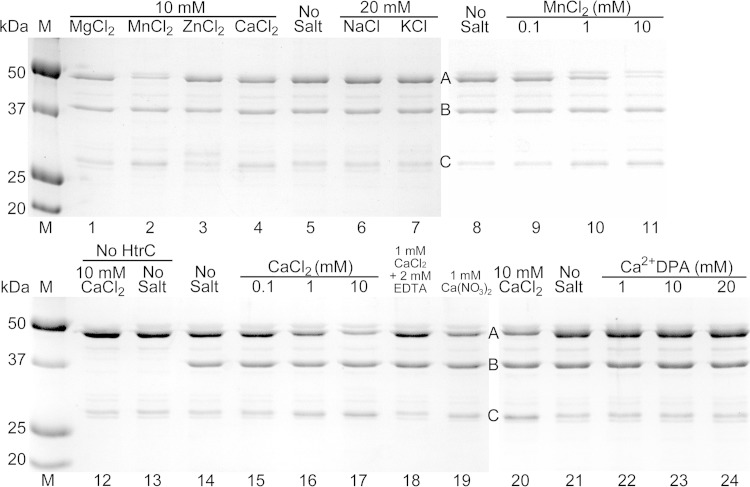
FIG 8.
In vitro cleavage of YpeB by HtrC. Purified YpeB21–446 (A) (6 μM) was incubated for 4 h with purified HtrC45–391 (B) (1 μM) unless otherwise indicated, with the indicated small-molecule additions. Reactions were terminated using SDS and heat. Samples were separated by SDS-PAGE, and proteins, including the YpeB cleavage product (C), were detected by staining with Coomassie brilliant blue. Protein concentrations were 12 μM YpeB21–446 and 2 μM HtrC45–391 in lanes 20 to 24. Lanes 1 to 7, 8 to 11, 12 to 19, and 20 to 24 are from four different gels. The masses of standard proteins (M) are indicated on the left.