Fig. 6.
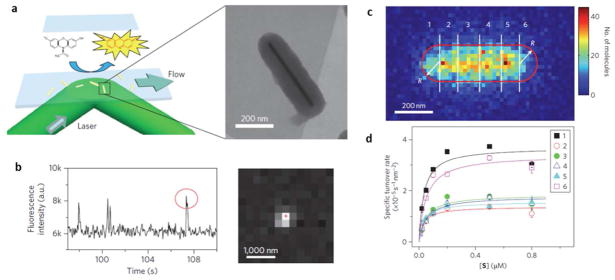
Super-localization of single fluorescent molecules reveals the reactivity of single nanoparticles. (a) The fluorogenic reaction converts a non-fluorescent molecule (reactant) to a fluorescent molecule (product). (b) Such single molecule reaction results in the appearance of a single molecule fluorescence pattern and a burst in the fluorescence emission at certain location. (c) By counting the number of single molecule reaction events across the Au nanorod, one can map the sub-particle distribution of reactivity. (d) Substrate concentration-dependent turn-over rate in six sub-particle regions as defined in (c) exhibited quite different reaction kinetics. Adapted by permission from Macmillan Publishers Ltd:[Nature Nanotechnology] (Ref. 104), copyright (2012).
