Abstract
Huanglongbing (HLB), presumably caused by the bacterium “Candidatus Liberibacter asiaticus,” is a devastating citrus disease associated with excessive preharvest fruit drop. Lasiodiplodia theobromae (diplodia) is the causal organism of citrus stem end rot (SER). The pathogen infects citrus fruit under the calyx abscission zone (AZ-C) and is associated with cell wall hydrolytic enzymes similar to plant enzymes involved in abscission. By means of DNA sequencing, diplodia was found in “Ca. Liberibacter asiaticus”-positive juice from HLB-symptomatic fruit (S) but not in “Ca. Liberibacter asiaticus”-negative juice. Therefore, the incidence of diplodia in fruit tissues, the impact on HLB-related postharvest decay, and the implications for HLB-related preharvest fruit drop were investigated in Hamlin and Valencia oranges. Quantitative PCR results (qPCR) revealed a significantly (P < 0.001) greater incidence of diplodia in the AZ-C of HLB-symptomatic (S; “Ca. Liberibacter asiaticus” threshold cycle [CT] of <30) than in the AZ-C of in asymptomatic (AS; “Ca. Liberibacter asiaticus” CT of ≥30) fruit. In agreement with the qPCR results, 2 weeks after exposure to ethylene, the incidences of SER in S fruit were 66.7% (Hamlin) and 58.7% (Valencia), whereas for AS fruit the decay rates were 6.7% (Hamlin) and 5.3% (Valencia). Diplodia colonization of S fruit AZ-C was observed by scanning electron microscopy and confirmed by PCR test and morphology of conidia in isolates from the AZ-C after surface sterilization. Diplodia CT values were negatively correlated with ethylene production (R = −0.838 for Hamlin; R = −0.858 for Valencia) in S fruit, and positively correlated with fruit detachment force (R = 0.855 for Hamlin; R = 0.850 for Valencia), suggesting that diplodia colonization in AZ-C may exacerbate HLB-associated preharvest fruit drop.
INTRODUCTION
Citrus huanglongbing (HLB; also known as citrus greening) is one of the most devastating diseases of citrus and has spread throughout the major citrus-producing regions in Asia, Africa, and the Americas, causing great losses for the citrus industry worldwide (1). HLB is associated with “Candidatus Liberibacter” spp., which are Gram-negative, phloem-limited bacteria (2, 3). The Asian form of HLB is currently present in the United States, and the causal pathogen, bacterium “Candidatus Liberibacter asiaticus,” was first confirmed in Southeast Florida in 2005 (1) and now is prevalent in all Florida citrus-growing areas in the state. HLB disease causes substantial economic loss by debilitating the productive capacity and shortening the life span of infected trees, promoting fruit drop and reducing fruit quality (4). Yield reduction can reach 30 to 100%, depending on the proportion of the canopy affected and the age of the trees during inoculation (1, 5).
HLB-diseased citrus plants develop a multitude of symptoms. Leaf symptoms include chlorosis, which commonly appears in an asymmetric pattern referred to as “blotchy mottle” (4). Chlorosis may typically appear on a single branch and subsequently spreads throughout the tree over time, especially on young trees; HLB-affected trees often show twig dieback. Fruit symptoms often manifest as a reduction in size and asymmetrical shape, and the fruit have curved central cores. Color development can be irregular and may only “break” on the stem end, leaving the majority of the fruit surface green. Brown discoloration may be present in the calyx abscission zone (AZ-C) located at the pedicel-fruit interface (4). HLB-affected fruit are located on HLB-symptomatic branches. Many fruit drop prematurely from affected branches, and this causes a reduced yield as the disease severity increases (5).
HLB not only affects plant health but can also alter the structure and composition of bacterial communities associated with citrus leaves (6), roots (7), and rhizospheres (8). In our investigation conducted to identify fungal species in orange juice from HLB-symptomatic (“Ca. Liberibacter asiaticus”-positive, S) fruit, the fungal pathogen Lasiodiplodia theobromae (synonyms: Diplodia natalensis, Botryodiplodia theobromae Pat., and Botryosphaeria rhodina), referred to here as diplodia, was consistently present in juice from S fruit but not in juice from healthy (“Ca. Liberibacter asiaticus”-negative) fruit.
Species of Lasiodiplodia, better known as diplodia, like other members of the family Botryosphaeriaceae, are pathogens with worldwide distribution in tropical and subtropical regions and cause different types of diseases in many woody plants, including fruit and tree crops such as mango, avocado, citrus, apple, peach, pear, Eucalyptus spp., azadirachta, and Pinus spp. (9–18). Infection of fruits by diplodia often leads to soft brown rot shortly before or after harvest (19, 20), while infection of trees causes distension and disruption of cell walls and thus weakens the strength of the wood (12). In citrus tree, there are reports that diplodia has been associated with “citrus gummosis,” resulting in the discoloration of tree bark and the exudation of gum (21) and twig blight or twig die back (in association with Colletrichum gleosporioides Penz.) (22). Diseases caused by Botryosphaeriaceae mostly follow the onset of some type of stress due to factors other than the Botryosphaeriaceae infection itself (23–26), so that Botryosphaeriaceae are generally referred to as endophytes and latent pathogens (12) but may act like saprophytes since they invade necrotic tissue (27).
In citrus fruits, invasion of the fruit tissue by diplodia can cause stem end rot (SER), which is typically a postharvest disease (18). The occurrence of SER is greatly enhanced by exposure to ethylene, which is commonly used to enhance the color development of the fruit (28, 29). Ethylene is a gaseous plant hormone that promotes chlorophyll degradation, ripening of climacteric fruits, senescence, and fruit abscission (30). Symptoms of diplodia SER on citrus fruit include rind softening around the button, followed by a brown discoloration of affected areas. Typically, decay develops at the stem end followed by the stylar end before involving the entire fruit, since the fungus progresses rapidly through the spongy central axis of the fruit, reaching the stylar end much sooner by this route than through the rind (31). In citrus fruits, diplodia infects the calyx and floral disk (button) during development. The infection then normally remains quiescent, while the fruit is on the tree, and the fungus does not usually invade the fruit until after harvest (27).
Like many other fruit-rotting fungi, diplodia has been reported to produce and secrete cell wall-digesting enzymes, including polygalacturonase, cellulase, and β-glucosidase (32–35), similar to citrus enzymes involved in abscission (36–38). In addition, it has been reported that the increased activity of the cell wall-digesting enzymes was associated with increased incidence of SER (39). One of the symptoms of HLB disease is excessive fruit drop, but the mechanism is not known, nor is HLB disease associated with increased ethylene production (40). Rosales and Burns (41) reported that fruit asymptomatic and symptomatic for HLB produced less ethylene than “healthy fruit” (harvested from “Ca. Liberibacter asiaticus”-negative trees). Preharvest fruit drop may result from fruit starvation due to HLB-related phloem impairment (42), the resulting nutritional deficits in the tree, and/or the reduced efficiency in absorbing water due to loss of roots (43). However, these speculations lack confirmation as of yet, and the enhanced nutritional programs adapted by growers to reduce tree disease symptoms have not stopped the fruit drop problems (44). In the last 2 years, the citrus preharvest fruit drop was the most severe ever recorded (45). Based on our preliminary data revealing a consistent presence of diplodia in HLB-affected fruit juice, as well as the relationship between diplodia and cell wall hydrolytic enzymes in the calyx abscission zone, it is possible that diplodia infection may contribute to the HLB-associated premature fruit drop. To address this, we investigated the incidence of diplodia infection, diplodia colonization of the calyx abscission zone (AZ-C), and the relationship between diplodia infection and fruit detachment force (FDF), as well as fruit ethylene production in HLB-symptomatic (S) and -asymptomatic (AS) fruit for two commercially important sweet orange (Citrus sinensis) cultivars (Hamlin and Valencia).
MATERIALS AND METHODS
Orange juice samples.
Juice was extracted from Valencia and Hamlin oranges and pasteurized under simulated commercial conditions (46) from multiple harvests over 4 years (2010 to 2013) from healthy (“Ca. Liberibacter asiaticus”-negative) or HLB-affected (“Ca. Liberibacter asiaticus” positive) trees as determined by quantitative real-time PCR (qPCR) (47). Juice from fruit harvested from healthy trees was “Ca. Liberibacter asiaticus” negative (healthy juice), and juice from HLB-symptomatic fruit harvested from HLB-affected trees was positive for “Ca. Liberibacter asiaticus” (HLB-affected juice), as determined by qPCR (48).
Fresh fruit material.
Fruit asymptomatic (AS) or symptomatic for HLB disease (S) were collected from Hamlin and Valencia sweet orange trees in a commercial orchard in Indian River County, Florida. Selection of AS and S fruit in the field was by visual observation. AS fruit were collected from trees showing no symptoms of HLB, whereas S fruit were collected from HLB-symptomatic branches or canopy sectors of an HLB-symptomatic tree (yellowing or blotchy mottled leaves and twig dieback); S fruit were smaller than AS fruit, poorly colored, and misshapen. The presence or absence of “Ca. Liberibacter asiaticus” was verified later by qPCR analysis as described below. All S fruit were “Ca. Liberibacter asiaticus” positive (threshold cycle [CT] values of 19 to 30, Fig. 1), while some of the AS fruit were “Ca. Liberibacter asiaticus” negative and some had “Ca. Liberibacter asiaticus” CT values of >30 (Fig. 1). Three harvests of Hamlin fruit between 2013 December and 2014 January; and three harvests of Valencia fruit between 2014 February and 2014 May were collected. Fruit stems were clipped 15 to 20 cm above the fruit and immediately transported to the laboratory, where the fruit detachment force (FDF) was measured. Fungal isolation and preparation of samples for electron microscopy were conducted within 2 h of harvest; measurement of ethylene production and DNA extraction were conducted within 48 h of harvest.
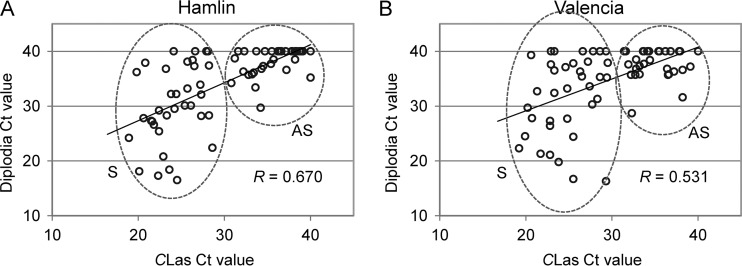
FIG 1.
Correlation of diplodia CT value and “Ca. Liberibacter asiaticus” CT value in Hamlin (A) and Valencia (B) fruit illustrated by Scatterplot. For those samples in which no amplification was detected, the CT value is shown as 40 in the chart. CLas, “Candidatus Liberibacter asiaticus”; S, huanglongbing-symptomatic; AS, asymptomatic.
DNA extraction.
With the stems already removed, the fruit side of the AZ-C plus the central fruit core (1 to 1.5 cm of tissue) was excised using a number 4 cork borer and then used for DNA extraction. Seventy fruit were used (35 for AS, 35 for S). DNA was extracted from 100 mg of plant tissue using a DNeasy plant minikit (Qiagen, Inc., Valencia, CA) according to manufacturer's instructions. DNA quality (260/280 and 260/230 ratios) and quantity were assessed by spectrophotometry (NanoDrop; Thermo Scientific, Waltham, MA).
Real-time PCR.
For “Ca. Liberibacter asiaticus” detection, the primers HLBasf and HLBr and the probe HLBp, with the following sequences, were used to target 16S rRNA genes of “Ca. Liberibacter asiaticus” (47): HLBas (F), TCGAGCGCGTATGCAATACG; HLBr (R), GCGTTATCCCGTAGAAAAAGGTAG; and HLBp (probe), 6-FAM-AGACGGGTGAGTAACGCG-MGBNFQ. For fungal detection, a set of universal fungal primers (UF-F and UF-R) was designed based on more than 50 fungi (filamentous fungi and yeasts) 18S rRNA gene sequences available from the NCBI database. They were designed to bind to two conserved regions encompassing a variable region (UF-F, CTCGTAGTTGAACCTTGG; UF-R, GCCTGCTTTGAACACTCT). For diplodia detection, specific primers targeting the diplodia β-tubulin gene (GenBank number DQ458858.1) were designed using software Primer Express 3.0.1 (TB-F, ATGGCTCCGGTGTGTAAGTGT; TB-R, TGCTACAGGTCAGCGATTGC). PCR mixtures with a total volume of 15 μl contained 7.5 μl of TaqMan PCR master mix or SYBR green PCR master mix (Applied Biosystems), 250 nM concentrations of each primer, 150 nM probe (for “Ca. Liberibacter asiaticus” detection), and 100 ng of template DNA. Real-time PCR amplifications were performed in a 7500 real-time PCR system (Applied Biosystems, Foster City, CA). The qPCR parameters were as follows: 95°C for 10 min, followed by 40 cycles at 95°C for 15 s and 60°C for 1 min, with fluorescence signal capture at each stage of 60°C. For SYBR green real-time PCR, the default melting curve (disassociation) stage is continued after the 40 cycles of PCR. The CT values were analyzed using ABI 7500 software version 2.0.6 (Applied Biosystems) with a manually set threshold at 0.02 and automated baseline settings.
PCR amplification, cloning, and sequencing of fungal 18S rRNA genes.
For DNA cloning, fungal 18S rRNA gene fragments were amplified by conventional PCR using the UF-F and UF-R primer set. PCR conditions were 12.5 μl of 2× GoTaq Green master mix (Promega), 300 nM concentrations of forward/reverse primer, and 1 μl of template DNA in a 25-μl reaction mixture. Temperature cycling was started with 95°C for 3 min, followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 60°C for 1 min, followed by a final extension at 72°C for 10 min. The PCR products were immediately ligated to TOPO TA vector pCR2.1 (Life Technologies, Carlsbad, CA) and transformed into chemically competent Escherichia coli TOP10 cells (Life Technologies). Transformants were grown on LB agar containing ampicillin (100 μg/ml). Colonies were screened using 40 μl of 2% (wt/vol) X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and 7 μl of 20% (wt/vol) IPTG (isopropyl-β-d-thiogalactopyranoside) per plate. White colonies were selected for analysis, and the presence of inserts was verified by PCR. Plasmid DNA was isolated from E. coli cultures by using a QIAprep spin miniprep kit (Qiagen), and DNA sequencing was performed at the U.S. Department of Agriculture/ARS U.S. Horticultural Research Laboratory Genomic Core Facility using the M13 universal primers and BigDye terminator version 3.1 and the 3730xl DNA analyzer (Applied Biosystems). Sequences were assembled by ContigExpress of Vector NTI (Invitrogen) and analyzed by AlignX in Vector NTI.
FDF.
The FDF was measured by using a force gauge (Force Five; Wagner Instruments, Greenwich, CT). Stem was clipped to ∼3 cm above the fruit, inserted into the gauge and fixed, and the fruit was then twisted and pulled until it separated from the stem. FDF is expressed in newtons (N).
Isolation of diplodia from the calyx abscission zone.
Immediately before processing, pedicels were clipped to ∼2 cm above the fruit and pulled from 6 AS and 6 S fruit. To eliminate superficial colonizers, pedicels with the AZ-C were initially washed with tap water, surface sterilized by immersion in 70% ethanol for 1 min, in a bleach solution containing 3.5% sodium hypochlorite for 2 min, and in 70% ethanol for 30 s, and finally rinsed three times (3 min each) in deionized and autoclaved water. The samples were then dried by blotting them on autoclaved paper towels. The stems were cut into 5-mm sections from the AZ-C side using an autoclaved razor blade, and then a thin layer (0.5 mm) of AZ-C surface was cut and removed. The sections were placed onto potato-dextrose agar (PDA), with the AZ-C side facing the medium. Cultures were kept on the laboratory bench at about 20 to 25°C for up to 30 days. The effectiveness of surface sterilization was checked by spreading 100 μl of the final rinse water on PDA, followed by incubation for 2 weeks at 20 to 25°C to confirm the absence of any microbial growth. The identities of the isolates were confirmed by PCR; fungi were subcultured on water agar supplemented with autoclaved citrus twigs on the agar surface to induce conidium formation.
Electron microscopy.
Pedicels were pulled from 6 AS and 6 S fruit immediately before processing and were cut (∼0.5-mm thickness) with a razor blade from the abscission zone side. The hand cut sections were dehydrated through a graded series of ethanol (30, 50, 70, 80, 90, and 95% [once for 30 min at each step]; 100% [three times for 1 h or overnight at each time]) and then immersed in a graded hexamethyldisilazane series prepared in ethanol (vol/vol; 30%, 50% [once for 30 min at each step], and 100% [twice for 1 h or overnight at each time]), air dried in a chemical hood, and then put into a 42°C oven overnight. The specimens were coated with gold/palladium using a Hummer Sputter Coater (Anatech USA, Union City, CA). Images were collected using a Hitachi S-4800 scanning electron microscope (Hitachi High Technologies America, Inc., Pleasanton, CA).
Ethylene production.
Ethylene production was determined for 35 AS and 35 S fruit by incubating individual fruit in 1-liter glass jars that were sealed for 1 h. One milliliter of headspace gas was withdrawn from each jar using a gas-tight syringe and analyzed for ethylene by gas chromatography (model 5890; Hewlett-Packard, Avondale, PA) equipped with a flame ionization detector and an activated alumina column.
Fruit decay assay.
For both Hamlin and Valencia oranges, AS and S fruit were divided at random into two groups of 25; one of each group was subjected to ethylene treatment in air containing ethylene at 10 μl/liter, 27.8°C, and 90 to 95% relative humidity for 4 days, and the second group (control) was held in air without ethylene at the same temperature and humidity as the ethylene-treated group. After ethylene treatment, the fruit were transferred to air at ambient temperature for 2 weeks, and the incidence of decay was recorded on days 3, 7, and 14 posttreatment.
Statistical analysis.
SAS version 9.3 (SAS Institute, Cary, NC) was used for analysis of the data. One-way analysis of variance (PROC ANOVA) at a 95% (P = 0.05) confidence interval was used to determine statistical significance of differences in diplodia CT values, decay rates, ethylene production, and our measurements between AS and S, where P < 0.05 was considered to be statistically significant. Pearson correlation (PROC CORR) was used to determine the linear correlations between the diplodia CT value, the “Ca. Liberibacter asiaticus” CT value, the FDF, and ethylene production.
RESULTS
Identification of diplodia in HLB-affected orange juice.
To investigate the microbial community of HLB-affected orange juice, universal fungal primers were used. And the PCR products obtained with universal fungal primers consistently showed different patterns of melting curves between healthy and HLB-affected juice. Melting curves of HLB-affected juice samples were characterized by a peak at 85.2°C, which could not be found in healthy juice samples (see Fig. S1A in the supplemental material), indicating different sequences were amplified from HLB-affected and healthy juice, although size of amplicons were not distinguishably different on an agarose gel (1.5%) (see Fig. S1B in the supplemental material). To identify fungi that were detected in HLB-affected but not healthy juice, PCR products from six healthy and six HLB-affected juice samples were cloned, and a single positive clone was randomly picked for each juice sample for subsequent sequencing. DNA sequences were BLAST searched in the NCBI database. Sequences amplified from four of six HLB-affected juice samples revealed 100% identity with the diplodia 18S rRNA gene sequence, but none of the clones from the 6 healthy juice samples had the diplodia sequence (see Table S1 in the supplemental material). Specific primers targeting diplodia β-tubulin gene were then used to validate the sequencing results, and the presence of diplodia was confirmed in 30 HLB-affected juice samples but in none of the 30 healthy juice samples.
Incidence of diplodia infection in HLB-affected sweet orange fruit revealed by qPCR analysis and decay rate assay.
A subsequent study was conducted using fruit from HLB-affected groves (there are few if any unaffected citrus groves in Florida at this time) using normal-looking AS fruit from trees asymptomatic for HLB disease versus HLB S fruit. The fruit side of AZ-C from 70 Hamlin (35 AS, 35 S) and 70 Valencia (35 AS, 35 S) oranges were analyzed by qPCR for diplodia (β-tubulin gene) and “Ca. Liberibacter asiaticus” (16S rRNA gene) detection. The “Ca. Liberibacter asiaticus” CT values for AS fruit were between 30.8 and 40 (Hamlin) and between 31.1 and 40 (Valencia), while for S fruit these values were between 18.9 and 28.6 (Hamlin) and between 19.2 and 29.5 (Valencia). The diplodia CT values for AS fruit were between 29.7 and 40 (Hamlin) and between 28.7 and 40 (Valencia), while for S fruit the values were between 16.5 and 40 (Hamlin) and between 16.3 and 40 (Valencia). As shown in Fig. 1, diplodia CT values were positively correlated with “Ca. Liberibacter asiaticus” CT values (R = 0.670 for Hamlin and R = 0.531 for Valencia; P < 0.0001). The results showed significantly lower diplodia CT values in S than in AS fruit (P < 0.001) for both Hamlin and Valencia oranges. The incidences of S fruit with diplodia CT values of <35 were 68.6 and 54.3% in Hamlin and Valencia oranges, respectively, while in AS fruit, these rates were 8.6 and 5.7%, respectively.
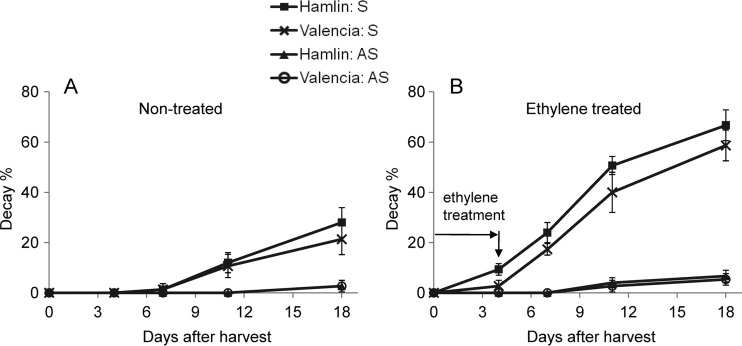
Diplodia infection is known to cause SER, and the incidence of SER is exacerbated by exposure to ethylene (28). Therefore, three batches of 100 Hamlin fruit (50 AS and 50 S) and 100 Valencia fruit (50 AS and 50 S) were used in a decay assay, with or without ethylene treatment. One of the batches of Hamlin fruit that was used in the decay rate assay is shown in Fig. S2 in the supplemental material. The S fruit had much higher decay rates than AS fruit (P < 0.001) (Fig. 2) for both air-treated control fruit and especially for ethylene-treated fruit. In agreement with the qPCR results, at 2 weeks after exposure to ethylene the incidences of SER in S fruit were as high as 66.7% (Hamlin) and 58.7% (Valencia), while for AS fruit, the decay rates were only 6.7% (Hamlin) and 5.3% (Valencia), indicating that the S fruit were more susceptible to SER than were the AS fruit, and the decay rates of ethylene-treated fruit were close to the incidence of S and AS fruit, with CT values of <35, as tested by qPCR.
FIG 2.
Incidence of decay in orange fruit during ethylene treatment and subsequent air storage for Hamlin and Valencia huanglongbing-symptomatic (S) and asymptomatic (AS) fruit.
Assessment of diplodia colonization of the fruit calyx abscission zone.
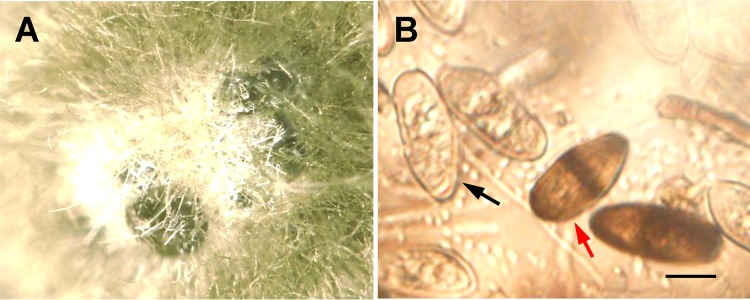
To assess diplodia colonization of the fruit abscission zone, fungi were isolated from AS and S fruit AZ-C tissues by using a procedure that has been used to isolate endophytes from inside plant tissues (49). The surface sterilization was effective, as indicated by the absence of any microbial growth on the medium after spreading and incubation for 2 weeks at room temperature of the final rinse water in the surface sterilization procedure. Isolates from AZ-C were analyzed by qPCR for diplodia identity, and the results indicated that diplodia was isolated from four of the six S fruit tissues, but not from any of the six AS fruit. To verify the specific primer for diplodia, the diplodia-positive fungi tested by qPCR were subcultured on autoclaved citrus twigs to induce characteristic conidia (see Fig. S3 in the supplemental material). Figure 3A shows the diplodia pycnidium formed in culture on the citrus twig, and Fig. 3B shows the conidia discharged from a pycnidium. Immature conidia are hyaline and unicellular, and the mature conidia are brown, dark walled, and one septate, findings consistent with the features and dimensions of the diplodia conidia documented in the literature (50).
FIG 3.
(A) Pycnidium formed in culture on citrus twig; (B) diplodia conidia discharged from the pycnidium. The black arrow indicates immature (nonseptate) conidia, and the red arrow indicates mature (septate) conidia. Scale bar, 10 μm.
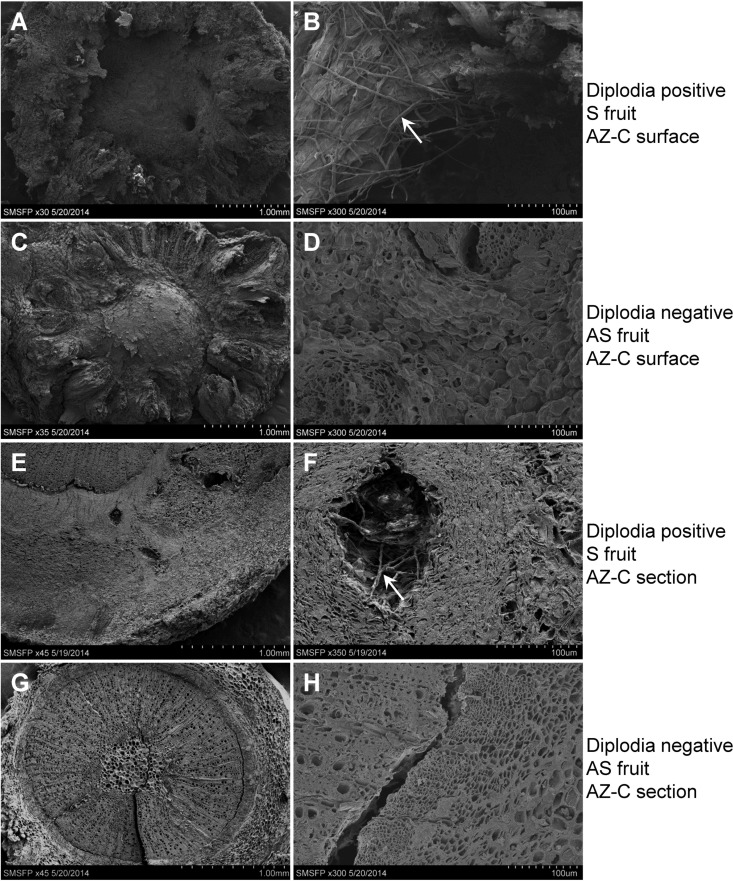
To further validate diplodia colonization of AZ-C, specimens from six S and six AS fruit were examined by scanning electron microscopy (SEM). Diplodia titers for these fruit were determined by qPCR (DNA was isolated from abscission zone fruit side). Three of the S fruit (out of six) detected diplodia with CT values of <30, and different sizes of cavities were found by SEM on the surface of AZ-C after pedicel was pulled from the fruit (Fig. 4A), as well as found in the section of the AZ-C specimen (Fig. 4E). Under higher magnifications, fungal hyphae were observed in the cavities (Fig. 4B and F), indicating diplodia colonization of the plant tissue. In contrast, none of the six AS fruit was detectable for diplodia by qPCR, and no fungal hyphae were found by SEM on the surface of the AZ-C (Fig. 4C and D) or in the specimen section (Fig. 4G and H).
FIG 4.
Scanning electron microscopy images of the calyx abscission zone (AZ-C). The surface of the AZ-C after pedicel was pulled from diplodia-positive, huanglongbing-symptomatic (S) fruit (A and B) and diplodia-negative, asymptomatic (AS) fruit (C and D) is shown. Sections of AZ-C from diplodia-positive, S fruit (E and F) and diplodia-negative, AS fruit (G and H) are also shown. The arrows in panels B and F indicate fungal hyphae. Panels B, D, F, and H are higher magnifications of panels A, C, E, and G, respectively. Magnifications: A, ×30; B, ×300; C, ×35; D, ×300; E, ×45; F, ×350; G, ×45; H, ×300.
Ethylene production in HLB-symptomatic orange fruit and its relationship with diplodia infection.
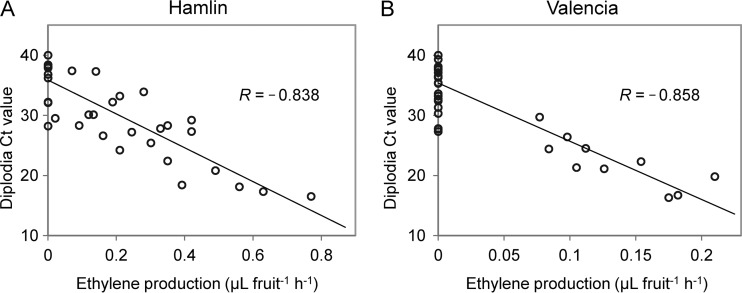
Since ethylene is an important plant hormone involved in abscission (40), fruit ethylene production was measured. No ethylene production was detected in any of the AS fruit under the methods used; however, in some of the S fruit, ethylene production was detectable, and generally, ethylene production was detected in those fruit that had diplodia CT values of <30. Statistical analysis revealed negative linear relationships between diplodia CT values and the amount of ethylene produced in both Hamlin (Pearson correlation coefficient = −0.838, P < 0.0001) (Fig. 5A) and Valencia (Pearson correlation coefficient = −0.858, P < 0.0001) oranges (Fig. 5B), indicating positive correlations between diplodia titers and ethylene production in both orange varieties. This is not surprising since the ethylene production rate was reported to increase as SER progressed in harvested Valencia oranges inoculated with diplodia (51).
FIG 5.
Correlation between diplodia CT values and ethylene production in huanglongbing-symptomatic Hamlin (A) and Valencia (B) fruit.
FDF and its relationship with diplodia infection in HLB-symptomatic orange fruit.
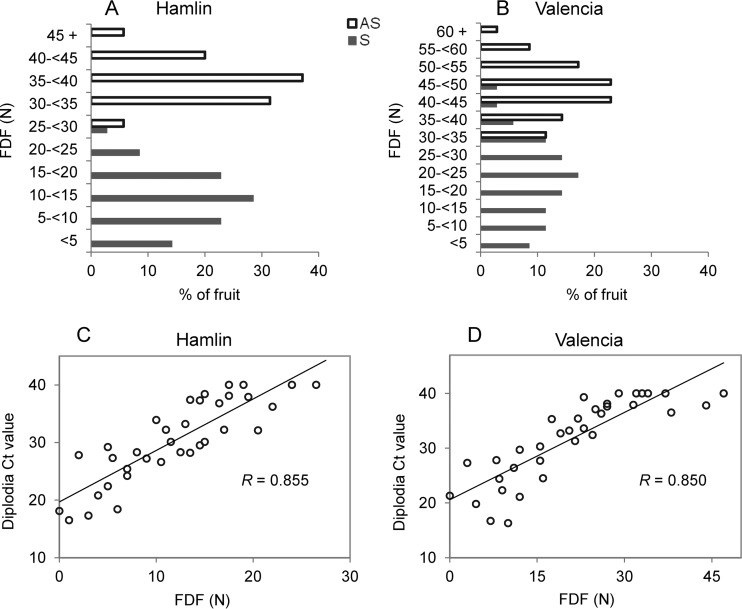
To find out the relationship between diplodia infection and FDF in HLB-affected fruit, FDF was measured and correlated to the diplodia CT values of individual S fruit. It was not surprising that the FDF for S fruit were generally lower than that for AS fruit. Figure 6 show the distributions of FDF among AS and S fruit for the two varieties. For Hamlin, the FDF for 88% of the S fruit was 0 to 20 N, whereas for AS fruit the FDF was 30 to 45 N. For Valencia, the FDF for 77% of AS fruit was between 35 and 55 N, while for S fruit FDF was in a broader range (0 to 50 N), with the majority (68.6%) between 10 and 35 N. Statistical analysis was used to analyze the correlation of FDF of S fruit and the diplodia CT values tested in the individual fruit. The results revealed positive linear correlations between diplodia CT values and FDF in both Hamlin (Pearson correlation coefficient = 0.855, P < 0.0001) and Valencia (Pearson correlation coefficient = 0.850, P < 0.0001) oranges (Fig. 6C and D), indicating negative correlations between diplodia titers and the FDF in both orange varieties.
FIG 6.
Distribution of fruit detachment force (FDF) in Hamlin (A) and Valencia (B) oranges and correlation between diplodia CT values and FDF in huanglongbing-symptomatic Hamlin (C) and Valencia (D) oranges. AS, asymptomatic; S, huanglongbing symptomatic.
DISCUSSION
Lasiodiplodia theobromae or diplodia, an opportunistic fungal pathogen in the citrus grove, has been known to infect citrus fruit by establishing in necrotic tissue on the button (calyx and floral disk) surface, but it does not normally start to colonize the fruit tissue until after harvest, when the button abscises and provides a temporary natural opening for penetration (27). The diplodia SER is rarely observed on normal mature fruit attached to the tree (31); thus, it is considered to be a postharvest problem. However, for S fruit, a high incidence of diplodia was noted inside the fruit tissue, on the fruit side of AZ-C, immediately upon harvest (indicating that it was present inside fruit tissue while the fruit were still on the tree), by both DNA-based molecular methods and by cell culture morphological methods. Diplodia colonization inside the AZ-C prior to harvest was further demonstrated by scanning electron microscopy. On HLB-affected trees, diplodia SER was even seen on fruit before harvest (see Fig. S4 in the supplemental material). Underlying the multitude of symptoms for HLB disease, there are dramatic physiological and anatomical changes which may influence the susceptibility of the host to other pathogens. According to the literature, the possible reasons responsible for the higher incidence of diplodia colonization in S orange fruit may include cell wall aberrations, compromised plant defense, and physiological disorders due to HLB disease. Swelling in the middle lamella, the collapse of cell walls, and separation of the adjacent cells were observed in “Ca. Liberibacter asiaticus”-infected citrus stems through transmission electron microscopy (52). Cell walls are not only the first line of plant defense protecting against pathogen penetration, they are also a source of signals used by plants to induce defense mechanisms (53). Anatomical changes in cell walls of HLB-affected stems may facilitate diplodia invasion of the abscission zone and fruit tissues. Although “Ca. Liberibacter asiaticus” elicits defense responses to some extent, they are very limited and delayed (42) and are not enough to suppress the HLB pathogen. In addition, it is noteworthy that “Ca. Liberibacter asiaticus” lacks type II plant cell wall-degrading enzymes (54), which have been known to play important roles in plant signaling to induce defense responses against fungal pathogens through autodegradation products of the plant cell wall (cell wall fragments or oligogalacturonides) (53). Moreover, studies have shown that the plant defense system is suppressed by HLB disease (42, 52, 55, 56). This weakened defense system in HLB-affected citrus trees could explain their increased susceptibility to other pathogens such as diplodia.
One of the major physiological disorders of citrus caused by HLB disease has been reported to be the massive starch accumulation in leaf and stem tissues due to aberrant carbohydrate metabolism and phloem blockage (57, 58). The tissues surrounding the fruit abscission zone are rich in vascular tissue where starch accumulation was found in HLB-affected citrus. The starch provides an excellent substrate for diplodia growth and colonization after entry, as suggested by Brown and Wilson (27), and starch in the abscission zones provided substrate for growth of diplodia after entry.
To summarize, the physiological disorder, cell wall aberration, and compromised plant defense may be responsible for the higher incidence of diplodia colonization in HLB-affected oranges and their abscission zones compared to healthy oranges.
As a consequence of the high incidence of diplodia infection, S Hamlin and Valencia oranges exhibited significantly higher postharvest decay rates than for their AS fruit. However, the impact of diplodia infection may not be limited to fruit decay. Diplodia colonization of the fruit AZ-C prior to the fruit harvest, and its effect on the FDF, may also impact preharvest fruit abscission, which should be investigated. Indeed, our study revealed a correlation between diplodia titer, FDF, and ethylene production. Since one of the symptoms of HLB disease is excessive preharvest fruit drop, the coincidence of diplodia infection and HLB disease, as well as the correlation between diplodia titer and the FDF, suggest that diplodia infection may contribute to the HLB-associated preharvest fruit drop.
Cell wall hydrolases such as cellulase, polygalacturonase, and pectinesterase are involved in abscission by modification and degradation of cell wall and the middle lamella of adjoining cells in the abscission zone. These enzymes are upregulated by the plant hormone ethylene (37, 59, 60). As a pathogen of woody plants, the production and secretion of cell wall-digesting enzymes, including polygalacturonase, cellulase, and β-glucosidase by diplodia, has been well documented (32, 34, 61, 62). These diplodia-produced enzymes could contribute to fruit abscission directly by degrading the cell wall and middle lamella in the abscission zone, and they may also indirectly impact fruit abscission by inducing the fruit to produce ethylene, which in turn induces the plant abscission zone to produce cell wall-degrading enzymes. It has been reported that the fungal pectolytic enzymes and their products induce ethylene in citrus (63) and tomato (64, 65) fruits.
Ethylene production was detected in some of the S fruit but was not detected in AS fruit using the methods described here. Generally, fruit from which ethylene production was detectable were also diplodia positive, as determined by qPCR, and the amount of ethylene production was negatively correlated with diplodia CT values. Since it was reported that ethylene stimulates rapid growth of diplodia and its invasion of tissue (66, 67), it possibly produces a snowball effect on fungal growth. Taken together, the impact of diplodia infection on fruit SER and possibly abscission may result from the combined effects of HLB, the fungal infection accompanied by cell wall-digesting enzymes, and the responses of fruit to the infection, including the production of ethylene. Brown and Burns (39) suggested that ethylene was needed to start the abscission zone forming for diplodia to enter, but in the case of HLB disease perhaps the HLB is causing a weakening at the abscission zone, allowing diplodia to enter and then to accelerate SER or even the abscission process.
In conclusion, a high incidence of diplodia colonization was found in S orange fruit compared to AS fruit and enhanced postharvest decay by SER. This will be a postharvest problem for the citrus fresh fruit industry. In addition, diplodia preharvest colonization of fruit AZ-C may exacerbate HLB-associated fruit drop, which should be explored. It has been reported that fungi causing fruit rot are generally recognized as initiating pathological fruit drop (68); diplodia and other fungi have been implicated for fruit drop of citrus (69), Shamouti orange (70), and Kinnow (71) fruits. The evidence for diplodia causing or exacerbating HLB-associated fruit drop from the present study is circumstantial at best since HLB disease has also been correlated with preharvest fruit drop, and it is difficult to separate the effects of the two diseases, which may also be synergistic. Interestingly, Rosales and Burns (41) found that HLB-affected fruit produced less ethylene than did healthy fruit, so HLB disease would seem not to induce abscission via ethylene, although Martinelli et al. (72) did find upregulation of some ethylene synthesis pathway enzymes and found ethylene signaling transcripts to be more abundant in S fruit than in healthy fruit, but they suggested that these genes do not necessarily affect ethylene levels. Nevertheless, the present study demonstrated a high incidence of preharvest diplodia colonization of HLB-affected sweet orange fruit tissues. The diplodia infection exacerbated postharvest fruit decay (SER) and may contribute to HLB-related preharvest fruit drop. Our finding implied that fungicide application to control diplodia may alleviate the HLB-associated preharvest fruit drop problem. Therefore, future studies will focus on fungal sprays targeting diplodia in the groves for their effect on fruit drop and SER, evidence of cell wall-digesting enzyme production associated with diplodia colonization, and the upregulation of ethylene pathway enzymes by diplodia in abscission zone tissue, as well as the relationship of FDF to fruit and juice quality.
Supplementary Material
ACKNOWLEDGMENTS
We thank James Salvatore for his assistance in harvesting fruit in the grove, Angela Ledger for assistance with cloning, Ric Stange for sequencing assistance, and Wayne Hunter for assistance in scanning electron microscopy.
Mention of a trademark or proprietary product is for identification only and does not imply a guarantee or warranty of the product by the U.S. Department of Agriculture.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.02972-14.
REFERENCES
- 1.Gottwald TR. 2010. Current epidemiological understanding of citrus Huanglongbing. Annu Rev Phytopathol 48:119–139. doi: 10.1146/annurev-phyto-073009-114418. [DOI] [PubMed] [Google Scholar]
- 2.Bastianel C, Garnier-Semancik M, Renaudin J, Bové J, Eveillard S. 2005. Diversity of “Candidatus Liberibacter asiaticus,” based on the omp gene sequence. Appl Environ Microbiol 71:6473–6478. doi: 10.1128/AEM.71.11.6473-6478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixeira DC, Saillard C, Eveillard S, Danet JL, da Costa PI, Ayres AJ, Bové J. 2005. ‘Candidatus Liberibacter americanus,’ associated with citrus huanglongbing (greening disease) in São Paulo State, Brazil. Int J Syst Evol Microbiol 55:1857–1862. doi: 10.1099/ijs.0.63677-0. [DOI] [PubMed] [Google Scholar]
- 4.Bové JM. 2006. Huanglongbing: a destructive, newly emerging, century-old disease of citrus. J Plant Pathol 88:7–37. [Google Scholar]
- 5.Bassanezi RB, Montesino LH, Gasparoto MCG, Bergamin Filho A, Amorim L. 2011. Yield loss caused by huanglongbing in different sweet orange cultivars in São Paulo, Brazil. Eur J Plant Pathol 130:577–586. doi: 10.1007/s10658-011-9779-1. [DOI] [Google Scholar]
- 6.Sagaram US, DeAngelis KM, Trivedi P, Andersen GL, Lu S-E, Wang N. 2009. Bacterial diversity analysis of Huanglongbing pathogen-infected citrus, using PhyloChip arrays and 16S rRNA gene clone library sequencing. Appl Environ Microbiol 75:1566–1574. doi: 10.1128/AEM.02404-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trivedi P, Duan Y, Wang N. 2010. Huanglongbing, a systemic disease, restructures the bacterial community associated with citrus roots. Appl Environ Microbiol 76:3427–3436. doi: 10.1128/AEM.02901-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trivedi P, He Z, Van Nostrand JD, Albrigo G, Zhou J, Wang N. 2012. Huanglongbing alters the structure and functional diversity of microbial communities associated with citrus rhizosphere. ISME J 6:363–383. doi: 10.1038/ismej.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma J, Mohanan C, Florence E. 1984. A new stem canker disease of Eucalyptus caused by Botryodiplodia theobromae in India. Trans Br Mycol Soc 83:162–163. doi: 10.1016/S0007-1536(84)80261-2. [DOI] [Google Scholar]
- 10.Verma K, Cheema S. 1984. Botryodiplodia theobromae: the cause of dieback and bark canker of pear in Punjab. Indian Phytopathol 37:325–327. [Google Scholar]
- 11.Mattos L, Ames T. 1986. Botryodiplodia theobromae, pathogenic on apple. Fitopatología 12:26–32. [Google Scholar]
- 12.Slippers B, Wingfield MJ. 2007. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology, and impact. Fungal Biol Rev 21:90–106. doi: 10.1016/j.fbr.2007.06.002. [DOI] [Google Scholar]
- 13.Phillips A, Lopes J, Abdollahzadeh J, Bobev S, Alves A. 2012. Resolving the Diplodia complex on apple and other Rosaceae hosts. Persoonia 29:29–38. doi: 10.3767/003158512X658899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Zhao L, Li G, Huang J, Hsiang T. 2011. Identification and characterization of Botryosphaeria spp. causing gummosis of peach trees in Hubei Province, central China. Plant Dis 95:1378–1384. doi: 10.1094/PDIS-12-10-0893. [DOI] [PubMed] [Google Scholar]
- 15.Shahbaz M, Iqbal Z, Saleem A, Anjum MA. 2009. Association of Lasiodiplodia theobromae with different decline disorders in mango (Mangifera indica L.). Pak J Bot 41:359–368. [Google Scholar]
- 16.Ismail A, Cirvilleri G, Polizzi G, Crous P, Groenewald J, Lombard L. 2012. Lasiodiplodia species associated with dieback disease of mango (Mangifera indica) in Egypt. Australas Plant Pathol 41:649–660. doi: 10.1007/s13313-012-0163-1. [DOI] [Google Scholar]
- 17.Luchi N, Longa O, Danti R, Capretti P, Maresi G. 2014. Diplodia sapinea: the main fungal species involved in the colonization of pine shoots in Italy. For Pathol 44:372–381. doi: 10.1111/efp.12109. [DOI] [Google Scholar]
- 18.Brown GE. 1986. Diplodia stem-end rot, a decay of citrus fruit increased by ethylene degreening treatment and its control. Proc Fla State Hortic Soc 99:105–108. [Google Scholar]
- 19.Johnson G, Mead A, Cooke A, Dean J. 1992. Mango stem end rot pathogens: fruit infection by endophytic colonization of the inflorescence and pedicel. Ann Appl Biol 120:225–234. [Google Scholar]
- 20.Kim KW, Park EW, Kim YH, Ahn K-K, Kim PG, Kim KS. 2001. Latency- and defense-related ultrastructural characteristics of apple fruit tissues infected with Botryosphaeria dothidea. Phytopathology 91:165–172. doi: 10.1094/PHYTO.2001.91.2.165. [DOI] [PubMed] [Google Scholar]
- 21.Assuah M, Oduro K, Ofuso-Budu K. 1999. Diplodia natalensis Pole Evans, the causal agent of citrus gummosis disease in Ghana. Ghana J Agric Sci 32:11–18. [Google Scholar]
- 22.Fawcett HS. 1936. Citrus diseases and their control. McGraw-Hill Book Co, Inc, New York, NY. [Google Scholar]
- 23.Blodgett J, Kruger E, Stanosz G. 1997. Sphaeropsis sapinea and water stress in a red pine plantation in central Wisconsin. Phytopathology 87:429–434. [DOI] [PubMed] [Google Scholar]
- 24.Schoeneweiss DF. 1981. The role of environmental stress in diseases of woody plants. Plant Dis 65:308–314. [Google Scholar]
- 25.Swart WJ, Wingfield MJ. 1991. Biology and control of Sphaeropsis sapinea on Pinus species in South Africa. Plant Dis 75:761–766. [Google Scholar]
- 26.Desprez-Loustau M-L, Marçais B, Nageleisen L-M, Piou D, Vannini A. 2006. Interactive effects of drought and pathogens in forest trees. Ann For Sci 63:597–612. doi: 10.1051/forest:2006040. [DOI] [Google Scholar]
- 27.Brown GE, Wilson W. 1967. Stem-end rot fungi in oranges: entry and possible use of pre-harvest fungicides for control. Proc Fla State Hortic Soc 80:301–305. [Google Scholar]
- 28.Barmore C, Brown G. 1985. Influence of ethylene on increased susceptibility of oranges to Diplodia natalensis. Plant Dis 69:228–230. [Google Scholar]
- 29.Sdiri S, Navarro P, Monterde A, Benabda J, Salvador A. 2012. New degreening treatments to improve the quality of citrus fruit combining different periods with and without ethylene exposure. Postharvest Biol Technol 63:25–32. doi: 10.1016/j.postharvbio.2011.08.005. [DOI] [Google Scholar]
- 30.Baldwin EA. 2004. Ethylene and postharvest commodities. HortScience 39:1538–1540. [Google Scholar]
- 31.Brown GE. 2000. Diplodia stem-end rot. University of Florida, Gainesville, FL: https://edis.ifas.ufl.edu/pdffiles/CH/CH10700.pdf. [Google Scholar]
- 32.Adisa V, Fajola A. 1983. Cellulolytic enzymes associated with the fruit rots of Citrus sinensis caused by Aspergillus aculeatus and Botryodiplodia theobromae. Z Allg Mikrobiol 23:283–288. [PubMed] [Google Scholar]
- 33.Al-Hindi RR. 2013. Cell wall-degrading enzymes of fruit spoilage fungi. Life Sci J 10:2456–2463. [Google Scholar]
- 34.Umezurike GM. 1979. The cellulolytic enzymes of Botryodiplodia theobromae Pat. Separation and characterization of cellulases and beta-glucosidases. Biochem J 177:9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pathak V, Srivastava D. 1969. Pectinolytic enzymes of Diplodia natalensis pole Evans. J Phytopathol 65:263–267. [Google Scholar]
- 36.Xie R, Deng L, Jing L, He S, Ma Y, Yi S, Zheng Y, Zheng L. 2013. Recent advances in molecular events of fruit abscission. Biol Plant 57:201–209. doi: 10.1007/s10535-012-0282-0. [DOI] [Google Scholar]
- 37.Wu Z, Burns JK. 2004. A β-galactosidase gene is expressed during mature fruit abscission of ‘Valencia’ orange (Citrus sinensis). J Exp Bot 55:1483–1490. doi: 10.1093/jxb/erh163. [DOI] [PubMed] [Google Scholar]
- 38.Burns JK, Lewandowski DJ, Nairn CJ, Brown G. 1998. Endo-1,4-β-glucanase gene expression and cell wall hydrolase activities during abscission in Valencia orange. Physiol Plant 102:217–225. [Google Scholar]
- 39.Brown GE, Burns JK. 1998. Enhanced activity of abscission enzymes predisposes oranges to invasion by Diplodia natalensis during ethylene degreening. Postharvest Biol Technol 14:217–227. [Google Scholar]
- 40.Brown KM. 1997. Ethylene and abscission. Physiol Plant 100:567–576. [Google Scholar]
- 41.Rosales R, Burns JK. 2011. Phytohormone changes and carbohydrate status in sweet orange fruit from Huanglongbing-infected trees. J Plant Growth Regul 30:312–321. doi: 10.1007/s00344-011-9193-0. [DOI] [Google Scholar]
- 42.Kim J-S, Sagaram US, Burns JK, Li J-L, Wang N. 2009. Response of sweet orange (Citrus sinensis) to “Candidatus Liberibacter asiaticus” infection: microscopy and microarray analyses. Phytopathology 99:50–57. doi: 10.1094/PHYTO-99-1-0050. [DOI] [PubMed] [Google Scholar]
- 43.Johnson E, Bright D, Graham J. 2012. Early root infection and damage in citrus huanglongbing disease development. Phytopathology 102:59–59. [Google Scholar]
- 44.Gottwald T, Graham J, Irey M, McCollum T, Wood B. 2012. Inconsequential effect of nutritional treatments on huanglongbing control, fruit quality, bacterial titer and disease progress. Crop Prot 36:73–82. doi: 10.1016/j.cropro.2012.01.004. [DOI] [Google Scholar]
- 45.USDA NASS. 2014. Citrus forecast (June 2014). USDA, National Agricultural Statistics Service, Washington, DC: http://www.nass.usda.gov/Statistics_by_State/Florida/Publications/Citrus/cit/2013-14/cit0614.pdf. [Google Scholar]
- 46.Baldwin E, Plotto A, Manthey J, McCollum G, Bai J, Irey M, Cameron R, Luzio G. 2010. Effect of Liberibacter infection (Huanglongbing disease) of citrus on orange fruit physiology and fruit/fruit juice quality: chemical and physical analyses. J Agric Food Chem 58:1247–1262. doi: 10.1021/jf9031958. [DOI] [PubMed] [Google Scholar]
- 47.Li W, Hartung JS, Levy L. 2006. Quantitative real-time PCR for detection and identification of Candidatus Liberibacter species associated with citrus huanglongbing. J Microbiol Methods 66:104–115. doi: 10.1016/j.mimet.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 48.Bai J, Baldwin E, Liao H-L, Zhao W, Kostenyuk I, Burns J, Irey M. 2013. Extraction of DNA from orange juice, and detection of bacterium Candidatus Liberibacter asiaticus by real-time PCR. J Agric Food Chem 61:9339–9346. doi: 10.1021/jf402364y. [DOI] [PubMed] [Google Scholar]
- 49.Guo L, Hyde K, Liew E. 2000. Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytol 147:617–630. doi: 10.1046/j.1469-8137.2000.00716.x. [DOI] [PubMed] [Google Scholar]
- 50.Phillips A, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald J, Crous P. 2013. The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167. doi: 10.3114/sim0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zauberman G, Barkai-Golan R. 1975. Changes in respiration and ethylene evolution induced by Diplodia natalensis in orange fruit. Phytopathology 65:216–217. [Google Scholar]
- 52.Aritua V, Achor D, Gmitter FG, Albrigo G, Wang N. 2013. Transcriptional and microscopic analyses of citrus stem and root responses to Candidatus Liberibacter asiaticus infection. PLoS One 8:e73742. doi: 10.1371/journal.pone.0073742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferreira RB, Monteiro S, Freitas R, Santos CN, Chen Z, Batista LM, Duarte J, Borges A, Teixeira AR. 2007. The role of plant defence proteins in fungal pathogenesis. Mol Plant Pathol 8:677–700. doi: 10.1111/j.1364-3703.2007.00419.x. [DOI] [PubMed] [Google Scholar]
- 54.Duan Y, Zhou L, Hall DG, Li W, Doddapaneni H, Lin H, Liu L, Vahling CM, Gabriel DW, Williams KP. 2009. Complete genome sequence of citrus huanglongbing bacterium, “Candidatus Liberibacter asiaticus” obtained through metagenomics. Mol Plant Microbe Interact 22:1011–1020. doi: 10.1094/MPMI-22-8-1011. [DOI] [PubMed] [Google Scholar]
- 55.Nwugo CC, Duan Y, Lin H. 2013. Study on citrus response to huanglongbing highlights a downregulation of defense-related proteins in lemon plants upon ‘Ca. Liberibacter asiaticus’ infection. PLoS One 8:e67442. doi: 10.1371/journal.pone.0067442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albrecht U, Bowman KD. 2008. Gene expression in Citrus sinensis (L.) Osbeck following infection with the bacterial pathogen Candidatus Liberibacter asiaticus causing Huanglongbing in Florida. Plant Sci 175:291–306. doi: 10.1016/j.plantsci.2008.05.001. [DOI] [Google Scholar]
- 57.Etxeberria E, Gonzalez P, Achor D, Albrigo G. 2009. Anatomical distribution of abnormally high levels of starch in HLB-affected Valencia orange trees. Physiol Mol Plant Pathol 74:76–83. doi: 10.1016/j.pmpp.2009.09.004. [DOI] [Google Scholar]
- 58.Fan J, Chen C, Brlansky R, Gmitter F Jr, Li ZG. 2010. Changes in carbohydrate metabolism in Citrus sinensis infected with ‘Candidatus Liberibacter asiaticus.’ Plant Pathol 59:1037–1043. doi: 10.1111/j.1365-3059.2010.02328.x. [DOI] [Google Scholar]
- 59.Kazokas WC, Burns JK. 1998. Cellulase activity and gene expression in citrus fruit abscission zones during and after ethylene treatment. J Am Soc Hortic Sci 123:781–786. [Google Scholar]
- 60.Li J, Zhu H, Yuan R. 2010. Profiling the expression of genes related to ethylene biosynthesis, ethylene perception, and cell wall degradation during fruit abscission and fruit ripening in apple. J Am Soc Hortic Sci 135:391–401. [Google Scholar]
- 61.Arinze A, Smith I. 1979. Production of a polygalacturonase complex by Botryodiplodia theobromae and its involvement in the rot of sweet potato. Physiol Plant Pathol 14:141–152. [Google Scholar]
- 62.Gaber M, Saleh O, El-Fotouh E. 1990. Pectolytic, cellulolytic and proteolytic activities of two isolates of Botryodiplodia theobromae Pat. Ann Agric Sci 35:445–457. [Google Scholar]
- 63.Baldwin EA, Biggs RH. 1988. Cell wall lysing enzymes and products of cell wall digestion elicit ethylene in citrus. Physiol Plant 73:58–64. [Google Scholar]
- 64.Baldwin E, Pressey R. 1988. Tomato polygalacturonase elicits ethylene production in tomato fruit. J Am Soc Hortic Sci 113:92–95. [Google Scholar]
- 65.Baldwin EA, Pressey R. 1990. Exopolygalacturonase elicits ethylene production in tomato. HortScience 25:779–780. [Google Scholar]
- 66.Brown GE, Lee HS. 1993. Interactions of ethylene with citrus stem-end rot caused by Diplodia natalensis. Phytopathology 83:1204–1208. [Google Scholar]
- 67.El-Kazzaz M, Sommer N, Kader A. 1983. Ethylene effects on in vitro and in vivo growth of certain postharvest fruit-infecting fungi. Phytopathology 73:998–1001. [Google Scholar]
- 68.Racskó J, Leite G, Petri J, Zhongfu S, Wang Y, Szabó Z, Soltész M, Nyéki J. 2007. Fruit drop: the role of inner agents and environmental factors in the drop of flowers and fruits. Int J Hortic Sci 13:13–23. [Google Scholar]
- 69.Chaudhary NA, Rehman MA, Aziz A. 1994. Causes of early citrus decline, p 86–90. Proceedings of the International Conference on Citriculture. [Google Scholar]
- 70.Minz G. 1946. Diplodia natalensis, its occurrence on flowers, button and stem-end of Shamouti orange, and its relation to stem-end rot and fruit drop. Palest J Bot 5:152–168. [Google Scholar]
- 71.Shaft MU, Khan SM, Rehman A. 2004. Studies on the pathological fruit drop in Kinnow, p 97–105. Proceedings of International Conference on Citriculture. [Google Scholar]
- 72.Martinelli F, Uratsu SL, Albrecht U, Reagan RL, Phu ML, Britton M, Buffalo V, Fass J, Leicht E, Zhao W. 2012. Transcriptome profiling of citrus fruit response to huanglongbing disease. PLoS One 7:e38039. doi: 10.1371/journal.pone.0038039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.