Abstract
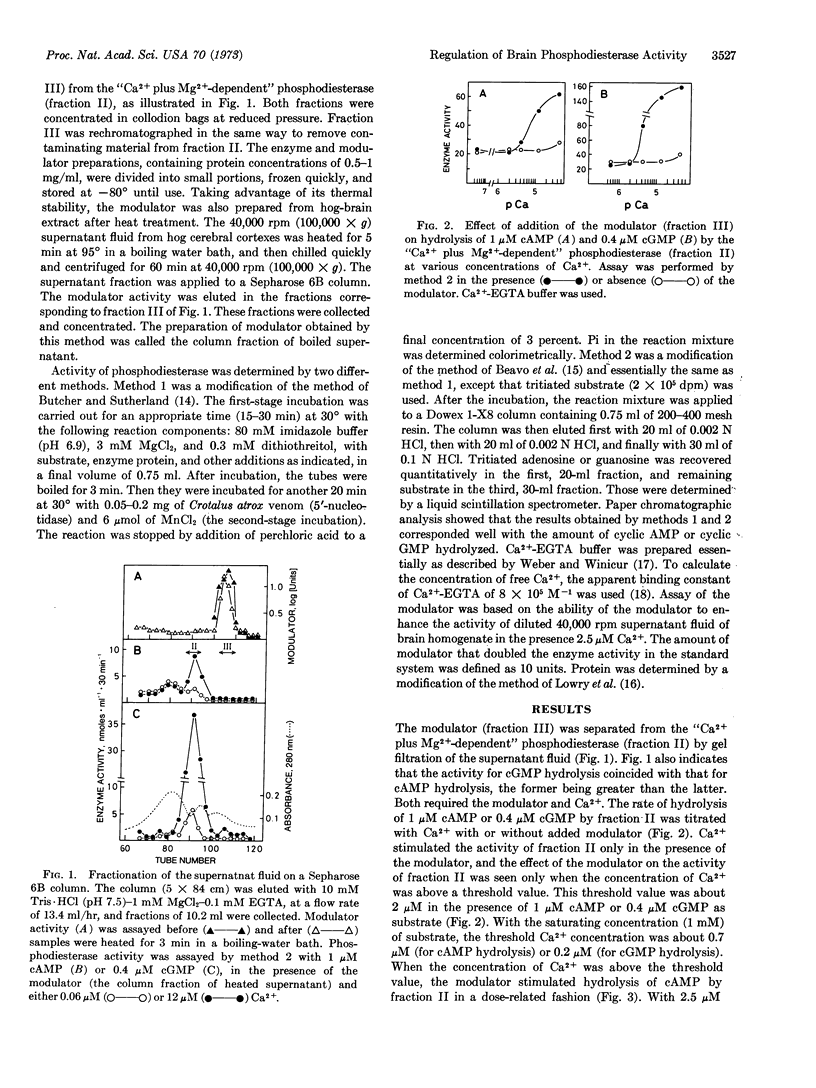
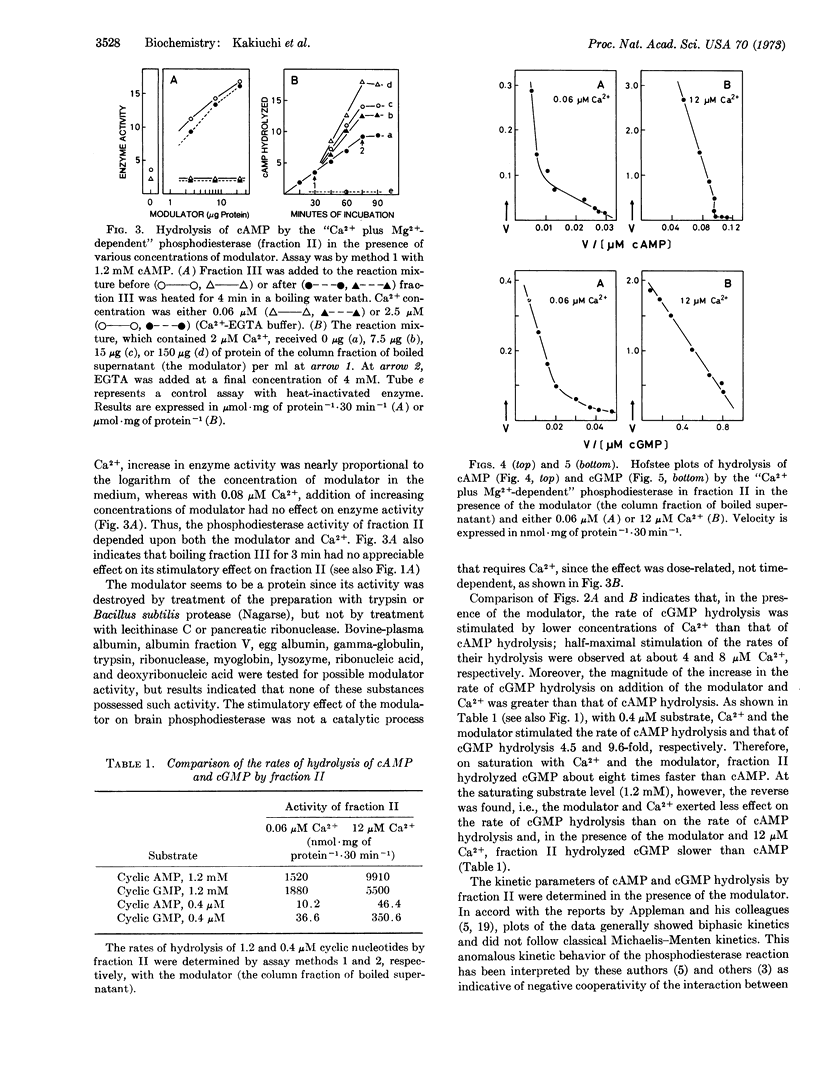
Gel filtration of the 40,000 rpm supernatant fraction of a homogenate of rat cerebral cortex on a Sepharose 6B column yielded two fractions: fraction II with the “Ca2+ plus Mg2+-dependent” phosphodiesterase activity and fraction III containing its modulator. The activity of fraction II was stimulated by micromolar concentrations of Ca2+ and the modulator when present together; the modulator stimulated the activity of fraction II only when the Ca2+ concentration was above a threshold value (about 2 μM with 0.4-1 μM substrate), and the stimulatory effect of Ca2+ was dependent upon the presence of the modulator. A possibility is discussed that the modulator may reversibly bind to the enzyme, which by itself is inactive, to form an active enzyme-modulator complex and that Ca2+ stimulates the activity of phosphodiesterase by shifting the equilibrium between these three species towards the formation of the active enzyme-modulator complex. Although fraction II hydrolyzed both cyclic AMP and cyclic GMP, hydrolysis of the latter was more significantly influenced by Ca2+ and the modulator than that of the former, and the “Ca2+ plus Mg2+-dependent” phosphodiesterase is likely to be a cyclic GMP enzyme. This conclusion is based on the following evidence: (a) Ca2+ stimulated hydrolysis of cyclic GMP by fraction II more than that of cyclic AMP. (b) In the presence of Ca2+ and the modulator, fraction II hydrolyzed cyclic GMP about 8 times faster than cyclic AMP when incubated with 0.4 μM substrate. (c) Half-maximal stimulation of hydrolysis of cyclic GMP was attained at a lower concentration of Ca2+ (4 μM) than that of cAMP (8 μM). (d) Increase in the concentration of Ca2+ from 0.06 μM to 12 μM in the presence of the modulator caused a decrease in the Km value of cyclic GMP hydrolysis by fraction II from 20 μM to 2 μM accompanied by 4-fold increase in the Vmax value. Under similar conditions, there was only a slight decrease in the Km value of cylic AMP hydrolysis (90 μM → 50 μM), although the Vmax value increased 7-fold. The anomalous shape of the kinetic plot of cyclic GMP hydrolysis became linear when the Ca2+ concentration was increased in the presence of the modulator. The modulator seems to be a protein, but it is heat stable. It is probably identical to the protein activator of phosphodiesterase first described by Cheung.
Keywords: rat cerebral cortex, Ca2+plus Mg2+-dependent phosphodiesterase, cyclic AMP, cyclic GMP, modulator protein
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTCHER R. W., SUTHERLAND E. W. Adenosine 3',5'-phosphate in biological materials. I. Purification and properties of cyclic 3',5'-nucleotide phosphodiesterase and use of this enzyme to characterize adenosine 3',5'-phosphate in human urine. J Biol Chem. 1962 Apr;237:1244–1250. [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Hydrolysis of cyclic guanosine and adenosine 3',5'-monophosphates by rat and bovine tissues. J Biol Chem. 1970 Nov 10;245(21):5649–5655. [PubMed] [Google Scholar]
- Beavo J. A., Hardman J. G., Sutherland E. W. Stimulation of adenosine 3',5'-monophosphate hydrolysis by guanosine 3',5'-monophosphate. J Biol Chem. 1971 Jun 25;246(12):3841–3846. [PubMed] [Google Scholar]
- Brooker G., Thomas L. J., Jr, Appleman M. M. The assay of adenosine 3',5'-cyclic monophosphate and guanosine 3',5'-cyclic monophosphate in biological materials by enzymatic radioisotopic displacement. Biochemistry. 1968 Dec;7(12):4177–4181. doi: 10.1021/bi00852a006. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- D'Armiento M., Johnson G. S., Pastan I. Regulation of adenosine 3',5'-cyclic monophosphate phosphodiesterase activity in fibroblasts by intracellular concentrations of cyclic adenosine monophosphate (3T3-dibutyryl cyclic AMP-SV40-transformed cells-michaelis constants-L cells-prostaglandin E 1 ). Proc Natl Acad Sci U S A. 1972 Feb;69(2):459–462. doi: 10.1073/pnas.69.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrendelli J. A., Steiner A. L., McDougal D. B., Jr, Kipnis D. M. The effect of oxotremorine and atropine on cGMP and cAMP levels in mouse cerebral cortex and cerebellum. Biochem Biophys Res Commun. 1970 Nov 25;41(4):1061–1067. doi: 10.1016/0006-291x(70)90193-2. [DOI] [PubMed] [Google Scholar]
- Franks D. J., Macmanus J. P. Cyclic GMP stimulation and inhibition of cyclic AMP phosphodiesterase from thymic lymphocytes. Biochem Biophys Res Commun. 1971 Mar 5;42(5):844–849. doi: 10.1016/0006-291x(71)90507-9. [DOI] [PubMed] [Google Scholar]
- George W. J., Polson J. B., O'Toole A. G., Goldberg N. D. Elevation of guanosine 3',5'-cyclic phosphate in rat heart after perfusion with acetylcholine. Proc Natl Acad Sci U S A. 1970 Jun;66(2):398–403. doi: 10.1073/pnas.66.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg N. D., Dietz S. B., O'Toole A. G. Cyclic guanosine 3',5'-monophosphate in mammalian tissues and urine. J Biol Chem. 1969 Aug 25;244(16):4458–4466. [PubMed] [Google Scholar]
- Goren E. N., Rosen O. M. The effect of nucleotides and a nondialyzable factor on the hydrolysis of cyclic AMP by a cyclic nucleotide phosphodiesterase from beef heart. Arch Biochem Biophys. 1971 Feb;142(2):720–723. doi: 10.1016/0003-9861(71)90540-6. [DOI] [PubMed] [Google Scholar]
- HOFSTEE B. H. J. On the evaluation of the constants Vm and KM in enzyme reactions. Science. 1952 Sep 26;116(3013):329–331. doi: 10.1126/science.116.3013.329. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Ishikawa S., Davis J. W., Sutherland E. W. Determination of guanosine 3',5'-monophosphate in tissues and of guanyl cyclase in rat intestine. J Biol Chem. 1969 Dec 10;244(23):6371–6376. [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3',5'-nucleotide phosphodiesterase (3). Biochem Biophys Res Commun. 1970 Dec 9;41(5):1104–1110. doi: 10.1016/0006-291x(70)90199-3. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y. Cyclic 3',5'-nucleotide phosphodiesterase, IV. Two enzymes with different properties from brain. Biochem Biophys Res Commun. 1971 Mar 5;42(5):968–974. doi: 10.1016/0006-291x(71)90525-0. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y. Regulation of brain phosphodiesterase activity: Ca++ plus Mg++ -dependent phosphodiesterase and its activating factor from rat brain. Adv Cyclic Nucleotide Res. 1972;1:455–477. [PubMed] [Google Scholar]
- Kuo J. F., Lee T. P., Reyes P. L., Walton K. G., Donnelly T. E., Jr, Greengard P. Cyclic nucleotide-dependent protein kinases. X. An assay method for the measurement of quanosine 3',5'-monophosphate in various biological materials and a study of agents regulating its levels in heart and brain. J Biol Chem. 1972 Jan 10;247(1):16–22. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ogawa Y. The apparent binding constant of glycoletherdiaminetetraacetic acid for calcium at neutral pH. J Biochem. 1968 Aug;64(2):255–257. doi: 10.1093/oxfordjournals.jbchem.a128887. [DOI] [PubMed] [Google Scholar]
- Russell T. R., Thompson W. J., Schneider F. W., Appleman M. M. 3':5'-cyclic adenosine monophosphate phosphodiesterase: negative cooperativity. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1791–1795. doi: 10.1073/pnas.69.7.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Pagliara A. S., Chase L. R., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. II. Adenosine 3',5'-monophosphate and guanosine 3',5'-monophosphate in mammalian tissues and body fluids. J Biol Chem. 1972 Feb 25;247(4):1114–1120. [PubMed] [Google Scholar]
- Teo T. S., Wang J. H. Mechanism of activation of a cyclic adenosine 3':5'-monophosphate phosphodiesterase from bovine heart by calcium ions. Identification of the protein activator as a Ca2+ binding protein. J Biol Chem. 1973 Sep 10;248(17):5950–5955. [PubMed] [Google Scholar]
- Teo T. S., Wang T. H., Wang J. H. Purification and properties of the protein activator of bovine heart cyclic adenosine 3',5'-monophosphate phosphodiesterase. J Biol Chem. 1973 Jan 25;248(2):588–595. [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Characterization of cyclic nucleotide phosphodiesterases of rat tissues. J Biol Chem. 1971 May 25;246(10):3145–3150. [PubMed] [Google Scholar]
- WEBER A., WINICUR S. The role of calcium in the superprecipitation of actomyosin. J Biol Chem. 1961 Dec;236:3198–3202. [PubMed] [Google Scholar]
- Wang J. H., Teo T. S., Wang T. H. Hysteretic substrate activation of bovine heart c-AMP phosphodiestrase. Biochem Biophys Res Commun. 1972 Feb 16;46(3):1306–1311. doi: 10.1016/s0006-291x(72)80117-7. [DOI] [PubMed] [Google Scholar]


