Abstract
AIM: To investigate the effects of terminal ileostomy on bacterial translocation (BT) and systemic inflammation after intestinal ischemia/reperfusion (I/R) injury in rats.
METHODS: Thirty-two rats were assigned to either the sham-operated group, I/R group, I/R + resection and anastomosis group, or the I/R + ileostomy group. The superior mesenteric artery was occluded for 60 min. After 4 h, tissue samples were collected for analysis. BT was assessed by bacteriologic cultures, intestinal permeability and serum levels of endotoxin; systemic inflammation was assessed by serum levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10, as well as by the activity of myeloperoxidase (MPO) and by intestinal histopathology.
RESULTS: Intestinal I/R injury not only caused morphologic damage to ileal mucosa, but also induced BT, increased MPO activity and promoted the release of TNF-α, IL-6, and IL-10 in serum. BT and ileal mucosa injuries were significantly improved and levels of TNF-α and IL-6 in serum were decreased in the I/R + ileostomy group compared with the I/R + resection and anastomosis group.
CONCLUSION: Terminal ileostomy can prevent the detrimental effects of intestinal I/R injury on BT, intestinal tissue, and inflammation.
Keywords: Bacterial reflux, Bacterial translocation, Intestinal ischemia/reperfusion, Terminal ileostomy
Core tip: Few studies have evaluated the occurrence of bacterial cecoileal reflux. We performed ileostomy to block the route of bacterial cecoileal reflux after intestinal ischemia/reperfusion injury. Compared with resection and anastomosis, ileostomy could improve the bacterial translocation, ileal mucosal injuries and systemic inflammation. The results provide a theoretic basis for the choice of ileostomy and resection/anastomosis in clinical practice.
INTRODUCTION
Bacterial translocation (BT) is an important factor affecting clinical prognosis when the body is under stress of any kind, such as trauma, burns or sepsis[1,2]. Previous studies have focused on the destruction of the intestinal mucosal barrier and the dysregulation of intestinal microbiota in disease[3-6], but have seldom focused on the importance of intestinal bacteria on reflux. Fritz et al[7] found that BT derives from the small bowel rather than from the colon. However, bacterial density in the small bowel is much lower than in the colon (104 and 107 bacteria/g in the jejunum and ileum, respectively, versus 1012 bacteria/g in the colon)[8]. The study by Berber et al[9] demonstrated that ileal bacterial counts increased approximately seven-fold following 24-h intestinal ischemia/reperfusion (I/R) injury. In addition, proliferation of the bacteria and the bacterial reflux from the cecum to the ileum could be important factors in small intestinal bacterial overgrowth, which is closely related to BT[10]. The occurrence of cecoileal reflux (CIR) during barium enema is a relatively common observation[11-13]. Perhaps due to difficulties in approaching the ileocecal junction, the most frequently adopted approach has been that of disregarding the importance of CIR and considering it as an irrelevant and common finding with no specific diagnostic meaning. Thus, there are few available data on the importance of CIR in BT.
Terminal ileostomy is one of the most common procedures in general surgery, and can disrupt the route of bacterial CIR. In the clinical situation, surgeons are often faced with a choice between ileostomy and primary anastomosis. Most previous studies comparing these two procedures were clinical trials, concerned mainly with morbidity and mortality[14-16]. Few studies have considered the influence of BT on the prognosis of patients. We postulate that ileostomy can interrupt the reflux of bacteria-rich colonic contents into the terminal ileum, thus alleviating bacterial overgrowth in the proximal intestine and improving BT in intestinal injury.
As a potentially important condition, intestinal I/R injury can be induced by traumatic, hemorrhagic or septic shock, acute mesenteric ischemia, severe burns, and some surgical procedures[17,18]. Previous studies suggested that intestinal I/R could induce injury of the intestinal mucosa following translocation of intestinal endotoxins and bacteria into blood, which may activate systemic inflammatory response syndrome and initiate multiple organ dysfunction syndrome[19]. An animal model of intestinal I/R injury is an established model for basic research, and intestinal ischemia caused by superior mesenteric artery (SMA) occlusion is stable and easy to repeat.
In this study, we investigated the protective effects of ileostomy against BT in a rat model of intestinal I/R injury and attempted to understand the intestinal and systemic inflammatory response following ileostomy and anastomosis, which could provide a theoretical basis for the choice of ileostomy and resection/anastomosis in clinical practice.
MATERIALS AND METHODS
Animals
Healthy adult male Sprague-Dawley rats (200-250 g) were obtained from Jinling Hospital, Nanjing, China. The rats were maintained in standard conditions with a 12 h light/dark cycle, controlled temperature (18 ± 4 °C), and appropriate humidity (about 50%). To maintain the integrity of the intestinal mucosa, the animals had free access to standard chow and water for one week. This study was approved by the Animal Care and Use Committee of the Medical School of Nanjing University, and all animals received care in accordance with the guide of the committee.
Operative procedure
Before the experiment, rats were fasted overnight, but had free access to water. The rat model of intestinal I/R injury was established according to a method described previously[20]. The rats were anesthetized with an injection of ketamine hydrochloride (100 mg/kg; ip). During the surgery, the rats were allowed to breathe spontaneously. Body temperature was maintained at approximately 37 °C using a heating pad. To prevent dehydration, Ringer’s lactate solution (10 mL/kg) was administered subcutaneously at the end of the operation. Prior to surgery, the abdomen was shaved and soaked twice with 10% povidine-iodine solution using sterile instruments. The abdomen was opened using a 4 cm midline incision, and the SMA was exposed. The rats were randomized into four groups: (1) sham-operated rats (sham-operated group; n = 8); (2) rats exposed to 60-min SMA occlusion followed by 4-h reperfusion (I/R group; n = 8); (3) rats exposed to I/R and then underwent partial intestinal resection and anastomosis (RA) (I/R + RA group; n = 8); and (4) rats exposed to I/R and then underwent terminal ileostomy (I/R + ileostomy group; n = 8) (Figure 1). In the sham-operated group, the SMA was isolated without occlusion. In the I/R, I/R + RA and I/R + ileostomy groups, the SMA was carefully isolated, and occluded at its origin with an atraumatic microvascular clamp for 60 min. The intestinal ischemia was confirmed by the loss of mesenteric pulsation and the intestines becoming pale. Reperfusion was confirmed by the return of mesenteric pulsation after removing the clamp. In the I/R group, the abdominal wall was immediately closed with a double-deck running suture. A 3 cm length of ileum at a point 2 cm proximal to the ileocecal valve was resected in the I/R + RA and I/R + ileostomy groups immediately after occluding the SMA. An end-to-end anastomosis was performed in the I/R + RA group, while in the I/R + ileostomy group, the end of the proximal ileum was brought out onto the abdominal wall as the ileostomy, and the distal end of the ileum was ligated. To avoid the bowel drying out, the abdominal cavity was irrigated with saline throughout the procedure.
Figure 1.
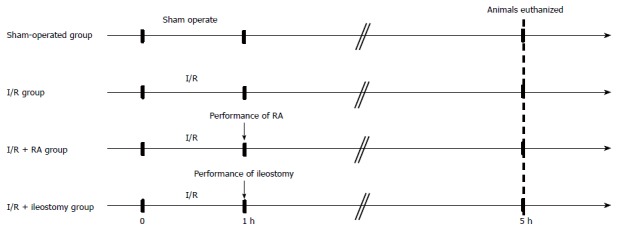
Experimental design. I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
Microbiologic analysis
All rats were anesthetized and euthanized after a 4-h reperfusion. The method used to perform microbiologic analysis was previously described[9]. Using an aseptic technique and sterile instruments, tissue samples from the liver, spleen, kidney, mesenteric lymph nodes (MLN), contents from the 10-cm terminal ileum and blood were taken for bacteriologic cultures. After being weighed, the ileal contents and tissue were stored in a sterile grinding tube. The tissues were homogenized in 1 mL saline. After diluting the homogenates, 0.1 mL dilutions were inoculated onto eosin methylene blue agar and blood agar, and incubated in ambient air for 24-48 h at 37 °C. Blood (0.5 mL) samples were cultured in 5 mL of brain-heart infusion broth for seven days at 37 °C. The cultures were checked daily, and sub-cultured on eosin methylene blue agar and blood agar plates. The number of colony-forming units/g of tissue homogenate was used to express the colonization.
Wet-to-dry weight ratio
Intestinal tissue samples (5 cm) were removed 15 cm proximal to the ileocecal valve and rinsed with saline. The wet weight of the intestine was determined and subsequently placed in a drying oven at 80 °C for 4 h. After desiccation, the tissue was weighed again to obtain the tissue dry weight. The ratio of the wet-to-dry (W/D) weight was calculated to provide an assessment of the extent of intestinal edema.
Assay of intestinal permeability
A modified method to assay the intestinal permeability was described previously[21]. At 4 h after reperfusion, we subjected additional rats from each group (n = 6 per group) to general anesthesia. After performing a midline laparotomy, a 5-cm-segment of distal ileum at a point 10 cm proximal to the ileocecal valve was isolated between silk ties. We injected a solution containing 25 mg of 4 kDa fluorescein isothiocyanate (FITC)-dextran diluted in 0.1 mL phosphate buffered saline into the enteric cavity. Then the bowel was put back in the abdominal cavity, and the abdomen was closed with a double-deck running suture. The animals were kept under general anesthesia. Thirty minutes after the injection of FITC-dextran, blood sample was collected by cardiac puncture and centrifuged at 10000 g for 10 min. Using a fluorescence spectrophotometer (F7000; Hitachi, Japan), the serous concentration of FITC-dextran was detected at excitation and emission wavelengths of 495 nm and 520 nm, respectively. By diluting serial serous concentrations of FITC-dextran, a standard curve was obtained.
Endotoxin and serum levels of TNF-α, IL-6 and IL-10 assays
After clotting for 60 min on ice, vena cava blood samples were centrifuged at 2500 × g for 10 min at 4 °C. Sera were obtained and stored at -70 °C. The serum levels of lipopolysaccharide were detected by colorimetric analysis for the limulus test (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s guidelines. The serum levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6 and IL-10 were measured by enzyme linked immunosorbent assay with commercially available kits (RD Systems, Minneapolis, MN, United States) according to the instructions of the manufacturer. Each reaction was performed twice.
Assay of myeloperoxidase activity
Myeloperoxidase (MPO) activity, which reflects polymorphonuclear neutrophil accumulation, was assessed by spectrophotometry. After weighing, intestinal tissue samples were homogenized on ice and MPO activity was measured quantitatively according to the manufacturer’s instructions (Jiancheng Biologic Project Company, Nanjing, China). Data are expressed as U/g wet weight, where one unit of MPO activity is defined by the conversion of 1 mmol H2O2 to H2O in one minute at 37 °C.
Histopathology
Segments of terminal ileum were excised, fixed for 48 h in 10% formalin and then embedded in paraffin. Sections 5 μm thick were cut and placed on microscope slides, and stained by hematoxylin and eosin. Images at magnification × 20 were obtained using a Zeiss Image A1 light microscope with Axiovision version4.5 software. Each slide was analyzed and reported by three pathologists who were all blinded to the source of the slides. A scale from 0 to 5 described by Chiu et al[22] was used to grade the intestinal mucosal lesions (Table 1).
Table 1.
Intestinal mucosal damage grading score
| Grade | Histologic characteristics |
| 0 | Normal mucosal villi |
| 1 | Development of subepithelial Gruenhagen’s space, usually at the apex of the villus, often with capillary congestion |
| 2 | Extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria |
| 3 | Massive epithelial lifting with a few denuded villi |
| 4 | Denuded villi with exposed dilated capillaries |
| 5 | Digestion and disintegration of lamina propria, hemorrhage, and ulceration |
Statistical analysis
Statistical analyses was performed with SPSS version 19.0 (IBM Corp., Armonk, NY, United States). Normally distributed data are shown as mean ± SD. χ2 or Fisher’s exact tests were used for the statistical evaluation of proportional comparisons for positive cultures of tissues. The remaining data were analyzed using one-way analysis of variance. Significant results were analyzed post hoc using the least significance difference test. Statistical significance was established as P < 0.05.
RESULTS
Bacterial translocation
During the experimental period, all the animals survived. In the I/R and I/R + RA groups, the incidence of BT to the liver, kidney, MLN and blood was significantly higher than in the sham-operated group (P < 0.05). However, the incidence of BT to the liver and MLN in the I/R + ileostomy group was significantly lower than in the I/R + RA group (P < 0.05). The I/R + ileostomy group and the sham-operated group only differed significantly for the incidence of BT to MLN (P < 0.05). In the I/R + ileostomy group, the counts of microorganisms cultured in the liver, spleen, kidney and MLN were significantly lower than in the I/R + RA group (P < 0.05; Table 2).
Table 2.
Incidence and number of bacterial translocations in tissue and blood samples
| Group (n = 8) |
Liver |
Spleen |
Kidney |
MLN |
Blood |
|||||
| Incidence | CFU | Incidence | CFU | Incidence | CFU | Incidence | CFU | Incidence | CFU | |
| Sham | 0/8 | 0 ± 0 | 0/8 | 0 ± 0 | 0/8 | 0 ± 0 | 0/8 | 0 ± 0 | 0/8 | 0 ± 0 |
| I/R | 6/8a | 841 ± 206.19a | 4/8 | 33 ± 13.39a | 5/8a | 261 ± 93.66a | 7/8a | 49750 ± 10592.70a | 5/8a | 36 ± 12.25a |
| I/R + RA | 7/8a | 1316 ± 236.22a | 6/8a | 61 ± 15.42a | 6/8a | 375 ± 88.74a | 8/8a | 64750 ± 8645.29a | 5/8a | 38 ± 11.84a |
| I/R + ileostomy | 2/8b | 171 ± 112.78b | 2/8 | 12 ± 7.69b | 3/8 | 68 ± 33.42b | 3/8ab | 4850 ± 2510.19b | 2/8 | 13 ± 5.40 |
CFU: Colony-forming units; I/R: Ischemia/reperfusion; MLN: Mesenteric lymph nodes; RA: Resection and anastomosis;
P < 0.05 vs sham;
P < 0.05 vs I/R + RA.
Ileal bacterial counts
Ileal bacterial counts in the I/R and I/R +RA groups were significantly higher than those in the sham-operated group (P < 0.01). Compared with the I/R + RA group, ileostomy significantly reduced the ileal bacterial counts after intestinal I/R injury (P < 0.01; Table 3).
Table 3.
Ileal bacterial counts
| Groups | Ileal bacterial count (CFU/g) |
| Sham | 82375 ± 10390.48 |
| I/R | 528750 ± 204341.28a |
| I/R + RA | 671250 ± 60280.22a |
| I/R + ileostomy | 177750 ± 21987.62b |
CFU: Colony-forming units; I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
P < 0.01 vs sham;
P < 0.01 vs I/R + RA.
W/D weight ratio
The intestinal W/D weight ratios in the I/R, I/R + RA and I/R + ileostomy groups were significantly higher than in the sham-operated group (P < 0.01). There were no significant differences in the intestinal W/D weight ratio between the I/R group and the I/R + RA group. The intestinal W/D weight ratio in I/R + ileostomy group was significantly lower than in the I/R + RA group (P < 0.01) (Figure 2).
Figure 2.
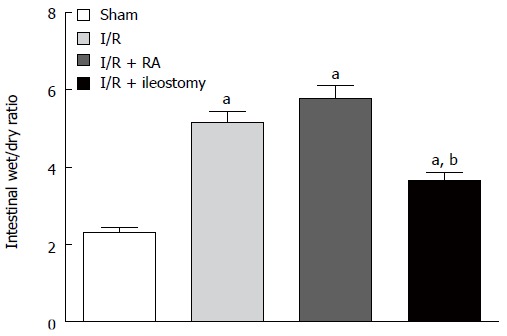
Wet-to-dry weight ratios. Data are expressed as mean ± SD. aP < 0.01 vs sham; bP < 0.01 vs I/R + RA. I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
Assay of intestinal permeability
Compared with the sham-operated group, the FITC-dextran levels detected in the serum were significantly higher after intestinal I/R injury (P < 0.01). Furthermore, RA or ileostomy did not significantly increase the amount of circulating FITC-dextran compared with the I/R group (Figure 3). These results indicate that RA or ileostomy did not increase intestinal permeability in I/R rats.
Figure 3.
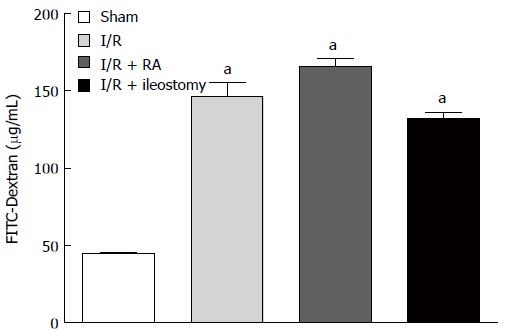
Fluorescein isothiocyanate-dextran levels in serum. Data are expressed as mean ± SD. aP < 0.01 vs sham. FITC: Fluorescein isothiocyanate; I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
Endotoxin assays
Intestinal I/R injury significantly increased the endogenous endotoxin levels compared with the sham-operated group. However, the endotoxin levels in the I/R + ileostomy were significantly lower than in the I/R + RA group (P < 0.01; Figure 4).
Figure 4.
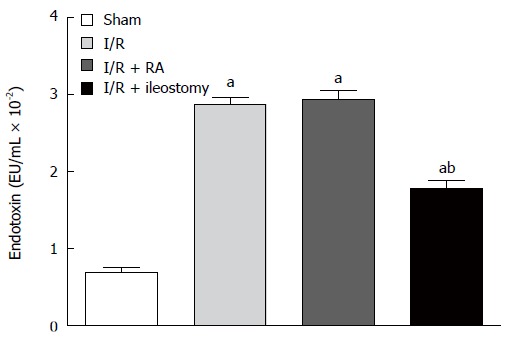
Endotoxin levels in serum. Data are expressed as mean ± SD. aP < 0.01 vs sham; bP < 0.01 vs I/R + RA. I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
Assay of TNF-α, IL-6 and IL-10 in serum
Compared with the sham-operated group, I/R significantly increased serum levels of the pro-inflammatory TNF-α (P < 0.01). RA after I/R injury increased the level of TNF-α in serum compared with I/R only rats, but was not significantly different. However, ileostomy after I/R injury did not increase serum TNF-α levels, which were significantly lower when compared with the I/R + RA group (P < 0.01). Similarly, the increases in serum IL-6 levels in the I/R, I/R + RA and I/R + ileostomy groups were significant as compared with sham-operated animals (P < 0.01). Ileostomy after I/R injury decreased serum IL-6 levels, which were significantly lower when compared with the I/R + RA group (P < 0.01). Serum IL-10 levels, as an indicator of anti-inflammatory response, were significantly higher in the I/R, I/R + RA and I/R + ileostomy groups than in the sham-operated animals (P < 0.01). Serum IL-10 levels in I/R, I/R + RA and I/R + ileostomy groups were not different (Figure 5).
Figure 5.
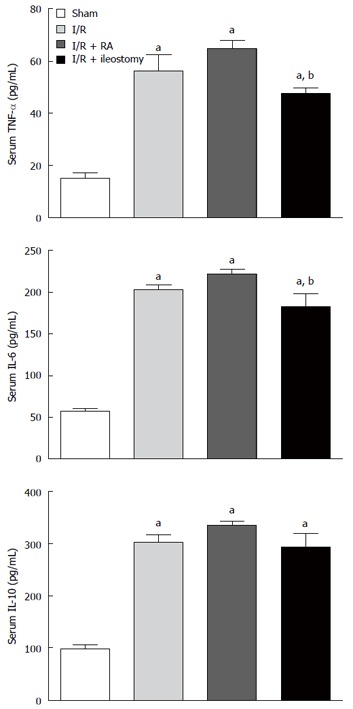
Serum levels of tumor necrosis factor tumor necrosis factor-α, -6 and IL-10 following intestinal ischemia/reperfusion injury in rats. Data are expressed as mean ± SD. aP < 0.01 vs sham; bP < 0.01 vs I/R + RA group. IL: Interleukin; TNF-α: Tumor necrosis factor-α; I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
Assay of MPO activity
Compared with the sham-operated group, MPO activity in the I/R and I/R + RA groups was significantly higher (P < 0.05). MPO activity in the I/R + ileostomy group was significantly lower than in the I/R group (P < 0.05), but higher than in the sham-operated group (P < 0.05) (Figure 6).
Figure 6.
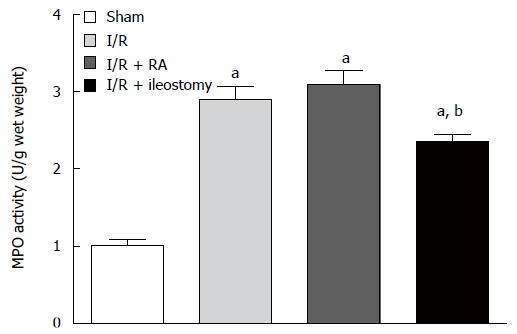
Myeloperoxidase activity in distal ileal tissue. Data are expressed as mean ± SD. aP < 0.05 vs sham; bP < 0.05 vs I/R + RA group. I/R: Ischemia/reperfusion; RA: Resection and anastomosis; MPO: Myeloperoxidase.
Histopathology
As shown in Figure 7, intestinal tissue segments from the sham-operated group showed normal morphology. Multiple erosions and massive infiltration of inflammatory cells in the lamina propria were observed after intestinal I/R injury. After I/R injury, damage to crypt cells and shortening of villi were typical histologic appearances. After intestinal I/R injury, atrophied mucosal tissue and leukocyte infiltration could be observed.
Figure 7.
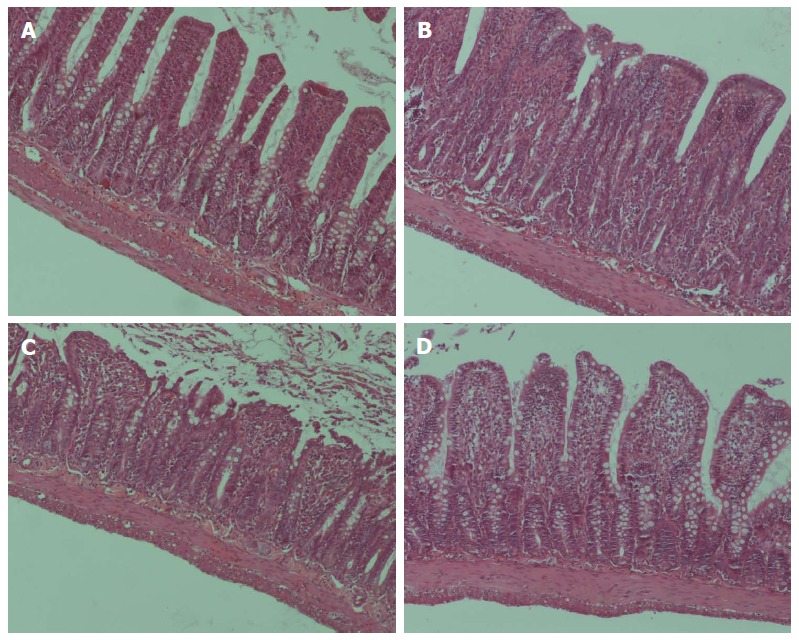
Light microscopic evaluation of the distal ileum. Hematoxylin and eosin staining in distal ileum (magnification × 40) in the A: sham-operated group, showing an intact mucosal barrier with normal lamina propria; B: Intestinal ischemia/reperfusion (I/R) injury resulted in acute mucosal damage; C: Resection and anastomosis did not ameliorate the mucosal damage caused by I/R injury; D: I/R + ileostomy resulted in an apparently intact mucosal barrier, though the epithelial cells appeared shrunken.
The Chiu’s histopathology score was lowest in the sham-operated group, and was highest in the I/R + RA group (P < 0.01). Although there was no significant difference in scores among the I/R, I/R + RA and I/R + ileostomy groups, the score in the I/R + ileostomy group was lower than in the I/R and I/R + RA groups (Figure 8).
Figure 8.
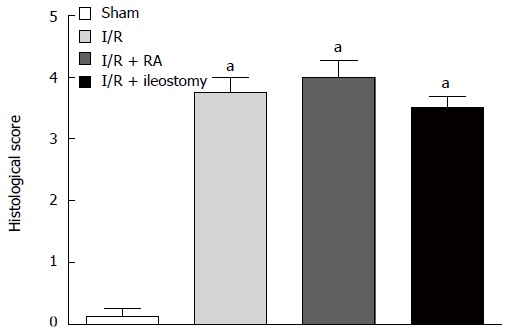
Histologic injury score of the intestines. Data are expressed as mean ± SD. aP < 0.01 vs sham. I/R: Ischemia/reperfusion; RA: Resection and anastomosis.
DISCUSSION
This is the first study to investigate BT in relation to ileostomy and intestinal resection/anastomosis, and the potential role of ileostomy in ameliorating BT and systemic inflammation in a rat model of intestinal I/R injury.
Physiologically, the intestinal barrier provides physical integrity and mucosal immunity[23]. Intestinal I/R injury can lead to the destruction of endothelial and epithelial cells and the breakdown of mucosal integrity, which results in increased intestinal permeability and dysfunction of the intestinal barrier[24]. Consequently indigenous intestinal bacteria translocate to other organs such as the blood, spleen, liver and MLN, leading to sepsis and multiple organ dysfunction syndrome[24]. In the present study, we found that construction of an ileostomy immediately after occlusion of the SMA significantly decreased the incidence of BT to the spleen, liver, kidney and MLN. Ileostomy also significantly reduced ileal bacterial overgrowth. Thus, ileostomy seems to have protective effects on the intestinal tract. Although it is not known how the ileostomy prevents translocation, we can hypothesize that the prevention of contamination by CIR appears to be related to the protective effect of ileostomy. It has been shown by previous studies that intestinal I/R could not only cause injury to mucosa, but also delay the gastrointestinal transit time[25-27]. At the same time, accumulating experimental evidence has shown the occurrence of ileal contamination due to reflux in experimental models or after surgical intervention in the ileocecal valve[28-30]. The occurrence of CIR during barium enema is a common observation[11-13]. These are refluxes of varying intensity that oscillate from small to large that can fill long ileal segments. The results of our experiment suggest that the protective effects of ileostomy on intestinal BT may be partly due to the prevention of contamination by CIR.
As the initial BT process, attachment of bacteria to the enterocyte can mediate the production of cytokines, resulting in an inflammatory response. Following the passage of bacteria through the epithelial barrier, pro-inflammatory factors are excessively released, resulting in the destruction of intestinal barrier integrity and further BT[31]. The present study suggests that the serum levels of TNF-α and IL-6, the pro-inflammatory cytokines, increased after 4-h intestinal I/R injury. These results are consistent with previous studies demonstrating that intestinal I/R injury has a large effect on the release of cytokines[31]. It has been demonstrated that increased mortality in animal models is relevant to the increase in TNF-α and IL-6 levels after intestinal I/R injury[32,33]. The degree of tissue injury and mortality after intestinal I/R injury is determined by the balance between TNF-α and IL-10[34]. We found that, when compared with RA, ileostomy did not increase the levels of IL-6 and TNF-α in serum after intestinal I/R injury. This may be due to the decreased incidence of BT observed in rats treated with ileostomy.
In addition to the indigenous bacterial translocation, the endotoxic translocation may also induce the dysfunction of other organs as a result of changes in intestinal function. Endotoxin could potently stimulate the release of cytokines, such as IL-6 and TNF-α. These mediators of inflammation play an important role in the pathogenesis of systemic inflammatory response syndrome[35] and multiple organ dysfunction syndrome[36]. This study demonstrated that ileostomy after intestinal I/R injury significantly decreased the serum level of endotoxin. We reasoned that the protective effect of ileostomy appears to be related to its ability to prevent the reflux of endotoxin-rich colonic contents into the ileum.
As an indicator of neutrophil accumulation, MPO activity is increased in intestinal I/R injury[9,37]. In this study, the reduction in neutrophil accumulation, which was shown by the significantly decreased MPO activity in the intestine of ileostomy-treated rats, seems to be a consequence of preventing BT. In the course of intestinal I/R injury, neutrophils play an important role[38]. After intestinal I/R injury, mediators such as bacteria and cytokines appears to stimulate the systemic activation of neutrophils[38]. Further inflammation and oxidative damage was then promoted by these activated neutrophils.
Our data showed that the construction of a terminal ileostomy after intestinal I/R injury partially reversed tissue damage, and decreased intestinal W/D weight ratio and the histologic injury score. In addition to the prevention of ileal contamination by CIR, possible explanations for these results might be that the terminal ileostomy promotes the ileal clearing function after intestinal I/R injury. However, due to a lack of appropriate studies, terminal ileum clearing delay in individuals with CIR cannot be confirmed, and it is not possible to determine whether such a delay may be bacteria-specific or extend to other contents. As noted in gastroesophageal reflux disease genesis[39,40], further studies focusing on the pathogenic implications of inadequate clearing of individuals with CIR are desirable. If this delay is confirmed, it could lead to prolonged exposure of the ileum to bacteria and metabolic products originating in the colon[41,42], resulting in harmful consequences to body balance.
Demonstrating the effects of interrupting CIR on BT was the main purpose of this research. However, the time when CIR appears after intestinal I/R injury is difficult to determine. Therefore, further studies are needed to define the optimal time to perform a terminal ileostomy. Another limitation of this study is that we did not use exact methodology to confirm the CIR. Isotope- or fluorescent-labeled bacteria could be used to conduct further experiments.
In conclusion, this study suggests that ileostomy ameliorates the detrimental effects of intestinal I/R injury on the intestine and systemic inflammation in rats. We speculate that bacterial CIR plays an important role in BT, and the beneficial effects of ileostomy, such as improved BT and systemic inflammation, are attributed mainly to the interruption of CIR.
COMMENTS
Background
Bacterial translocation (BT) is an important factor affecting clinical prognosis when the body is under stress of any kind, including trauma, burns or sepsis. Previous studies paid little attention to the importance of the reflux of intestinal bacteria. Terminal ileostomy is one of the most common procedures in general surgery, which can block the route of bacterial cecoileal reflux (CIR). In clinical practice, surgeons often face the choice between ileostomy and resection/anastomosis. In this study, the authors investigated the protective effect of ileostomy against BT in a rat model of intestinal ischemia/reperfusion (I/R) injury to provide a theoretic basis for this surgery.
Research frontiers
The authors demonstrated that ileostomy could prevent the detrimental effects of intestinal I/R injury on the intestinal tissue and systemic inflammation in a rat model of superior mesenteric artery occlusion. They speculate that bacterial CIR plays an important role in BT, and the beneficial effects of ileostomy, such as the improved BT and systemic inflammatory, are attributed mainly to the interception of CIR.
Innovations and breakthroughs
Recent reports have highlighted the importance of BT in intestinal I/R injury in rats. Few studies have described the bacterial CIR during intestinal I/R injury. This is believed to be the first study to report that ileostomy could prevent the detrimental effects of intestinal I/R injury on BT, intestinal tissue, and inflammation. This study also provided theoretic gist for the choice of ileostomy and resection/anastomosis in clinical practice.
Applications
Based on the findings in this study that ileostomy can prevent the detrimental effects of intestinal I/R injury on the intestinal tissue and systemic inflammation in a rat model of superior mesenteric artery occlusion, ileostomy may be performed as a more suitable therapeutic option than resection/anastomosis to prevent BT after intestinal I/R injury, which requires exploratory laparotomy.
Terminology
CIR is a relatively common observation in barium enema. The refluxes are of varying intensity that oscillate from small to large, which can fill long ileal segments. The hypothesis of CIR innocuousness seems, however, rather implausible as it would differ from the behavior of refluxes in other topographies of the digestive system, where they exhibit a high complication potential, generating such frequent and repercussive diseases as the gastroesophageal reflux disease. What is actually observed is that, perhaps due to the difficulties in approaching the ileocecal junction, the most frequently adopted conduct has been that of disregarding the importance of CIR and considering it as an irrelevant and common finding with no specific diagnostic meaning.
Peer review
The study of Lin and coworkers clearly shows how ileostomy may contribute to preventing the detrimental effects of ligating the superior mesenteric artery. The paper is well designed, the conclusions are supported by specific data, and its length and bibliography are appropriate.
Footnotes
Supported by National Natural Science Foundation of China No. 81270884, the 12th Five-Year Plan major project of PLA No. AWS12J001, and Jiangsu Province’s Key Medical Talent Program of China No. RC2011128
P- Reviewer: Arai M, Ciccocioppo R, Sinha R S- Editor: Qi Y L- Editor: AmEditor E- Editor: Zhang DN
References
- 1.Macpherson AJ, Harris NL. Interactions between commensal intestinal bacteria and the immune system. Nat Rev Immunol. 2004;4:478–485. doi: 10.1038/nri1373. [DOI] [PubMed] [Google Scholar]
- 2.O’Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacFie J, O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45:223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemaire LC, van Lanschot JJ, Stoutenbeek CP, van Deventer SJ, Wells CL, Gouma DJ. Bacterial translocation in multiple organ failure: cause or epiphenomenon still unproven. Br J Surg. 1997;84:1340–1350. [PubMed] [Google Scholar]
- 5.Honda K, Takeda K. Regulatory mechanisms of immune responses to intestinal bacteria. Mucosal Immunol. 2009;2:187–196. doi: 10.1038/mi.2009.8. [DOI] [PubMed] [Google Scholar]
- 6.Hofer U, Speck RF. Disturbance of the gut-associated lymphoid tissue is associated with disease progression in chronic HIV infection. Semin Immunopathol. 2009;31:257–266. doi: 10.1007/s00281-009-0158-3. [DOI] [PubMed] [Google Scholar]
- 7.Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, Will-Schweiger K, Kommerell M, Büchler MW, Werner J. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200:111–117. doi: 10.1016/j.amjsurg.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- 9.Berber I, Aydin C, Cevahir N, Yenisey C, Gumrukcu G, Kocbil G, Tellioglu G, Tekin K. Tempol reduces bacterial translocation after ischemia/reperfusion injury in a rat model of superior mesenteric artery occlusion. Surg Today. 2009;39:407–413. doi: 10.1007/s00595-008-3900-x. [DOI] [PubMed] [Google Scholar]
- 10.Grace E, Shaw C, Whelan K, Andreyev HJ. Review article: small intestinal bacterial overgrowth--prevalence, clinical features, current and developing diagnostic tests, and treatment. Aliment Pharmacol Ther. 2013;38:674–688. doi: 10.1111/apt.12456. [DOI] [PubMed] [Google Scholar]
- 11.Miller RE. Complete reflux small bowel examination. Radiology. 1965;84:457–463. doi: 10.1148/84.3.457. [DOI] [PubMed] [Google Scholar]
- 12.Machado W, Morceli J. Prevalência, classificação e características do refluxo cecoileal diagnosticado pelo enema opaco. Radiol Bras. 2006;39:107–111. [Google Scholar]
- 13.Decarlo J. Complications associated with diagnostic barium enema. Surgery. 1960;47:965–969. [PubMed] [Google Scholar]
- 14.Jiménez Fuertes M, Costa Navarro D. Resection and primary anastomosis without diverting ileostomy for left colon emergencies: is it a safe procedure? World J Surg. 2012;36:1148–1153. doi: 10.1007/s00268-012-1513-4. [DOI] [PubMed] [Google Scholar]
- 15.Weinberg JA, Griffin RL, Vandromme MJ, Melton SM, George RL, Reiff DA, Kerby JD, Rue LW. Management of colon wounds in the setting of damage control laparotomy: a cautionary tale. J Trauma. 2009;67:929–935. doi: 10.1097/TA.0b013e3181991ab0. [DOI] [PubMed] [Google Scholar]
- 16.Ott MM, Norris PR, Diaz JJ, Collier BR, Jenkins JM, Gunter OL, Morris JA. Colon anastomosis after damage control laparotomy: recommendations from 174 trauma colectomies. J Trauma. 2011;70:595–602. doi: 10.1097/TA.0b013e31820b5dbf. [DOI] [PubMed] [Google Scholar]
- 17.Riddington DW, Venkatesh B, Boivin CM, Bonser RS, Elliott TS, Marshall T, Mountford PJ, Bion JF. Intestinal permeability, gastric intramucosal pH, and systemic endotoxemia in patients undergoing cardiopulmonary bypass. JAMA. 1996;275:1007–1012. [PubMed] [Google Scholar]
- 18.Cicalese L, Sileri P, Green M, Abu-Elmagd K, Kocoshis S, Reyes J. Bacterial translocation in clinical intestinal transplantation. Transplantation. 2001;71:1414–1417. doi: 10.1097/00007890-200105270-00010. [DOI] [PubMed] [Google Scholar]
- 19.Leaphart CL, Tepas JJ. The gut is a motor of organ system dysfunction. Surgery. 2007;141:563–569. doi: 10.1016/j.surg.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Lane JS, Todd KE, Lewis MP, Gloor B, Ashley SW, Reber HA, McFadden DW, Chandler CF. Interleukin-10 reduces the systemic inflammatory response in a murine model of intestinal ischemia/reperfusion. Surgery. 1997;122:288–294. doi: 10.1016/s0039-6060(97)90020-9. [DOI] [PubMed] [Google Scholar]
- 21.Chen LW, Wang JS, Hwang B, Chen JS, Hsu CM. Reversal of the effect of albumin on gut barrier function in burn by the inhibition of inducible isoform of nitric oxide synthase. Arch Surg. 2003;138:1219–1225. doi: 10.1001/archsurg.138.11.1219. [DOI] [PubMed] [Google Scholar]
- 22.Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478–483. doi: 10.1001/archsurg.1970.01340280030009. [DOI] [PubMed] [Google Scholar]
- 23.Laissue JA, Gebbers JO. The intestinal barrier and the gut-associated lymphoid tissue. Curr Stud Hematol Blood Transfus. 1992;(59):19–43. doi: 10.1159/000429607. [DOI] [PubMed] [Google Scholar]
- 24.Berg RD. Bacterial translocation from the gastrointestinal tract. Adv Exp Med Biol. 1999;473:11–30. doi: 10.1007/978-1-4615-4143-1_2. [DOI] [PubMed] [Google Scholar]
- 25.Udassin R, Eimerl D, Schiffman J, Haskel Y. Postischemic intestinal motility in rat is inversely correlated to length of ischemia. An in vivo animal model. Dig Dis Sci. 1995;40:1035–1038. doi: 10.1007/BF02064193. [DOI] [PubMed] [Google Scholar]
- 26.Hebra A, Brown MF, McGeehin K, Broussard D, Ross AJ. The effects of ischemia and reperfusion on intestinal motility. J Pediatr Surg. 1993;28:362–365; discussion 28: 365-366. doi: 10.1016/0022-3468(93)90232-a. [DOI] [PubMed] [Google Scholar]
- 27.Cattaruzza F, Cenac N, Barocelli E, Impicciatore M, Hyun E, Vergnolle N, Sternini C. Protective effect of proteinase-activated receptor 2 activation on motility impairment and tissue damage induced by intestinal ischemia/reperfusion in rodents. Am J Pathol. 2006;169:177–188. doi: 10.2353/ajpath.2006.051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richardson JD, Griffen WO. Ileocecal valve substitutes as bacteriologic barriers. Am J Surg. 1972;123:149–153. doi: 10.1016/0002-9610(72)90325-x. [DOI] [PubMed] [Google Scholar]
- 29.Myrvold H, Tindel MS, Isenberg HD, Stein TA, Scherer J, Wise L. The nipple valve as a sphincter substitute for the ileocecal valve: prevention of bacterial overgrowth in the small bowel. Surgery. 1984;96:42–47. [PubMed] [Google Scholar]
- 30.Griffen WO, Richardson JD, Medley ES. Prevention of small bowel contamination by ileocecal valve. South Med J. 1971;64:1056–1058. doi: 10.1097/00007611-197109000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Grotz MR, Deitch EA, Ding J, Xu D, Huang Q, Regel G. Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg. 1999;229:478–486. doi: 10.1097/00000658-199904000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamamoto S, Tanabe M, Wakabayashi G, Shimazu M, Matsumoto K, Kitajima M. The role of tumor necrosis factor-alpha and interleukin-1beta in ischemia-reperfusion injury of the rat small intestine. J Surg Res. 2001;99:134–141. doi: 10.1006/jsre.2001.6106. [DOI] [PubMed] [Google Scholar]
- 33.Grotz MR, Ding J, Guo W, Huang Q, Deitch EA. Comparison of plasma cytokine levels in rats subjected to superior mesenteric artery occlusion or hemorrhagic shock. Shock. 1995;3:362–368. [PubMed] [Google Scholar]
- 34.Souza DG, Teixeira MM. The balance between the production of tumor necrosis factor-alpha and interleukin-10 determines tissue injury and lethality during intestinal ischemia and reperfusion. Mem Inst Oswaldo Cruz. 2005;100 Suppl 1:59–66. doi: 10.1590/s0074-02762005000900011. [DOI] [PubMed] [Google Scholar]
- 35.Schietroma M, Carlei F, Mownah A, Franchi L, Mazzotta C, Sozio A, Amicucci G. Changes in the blood coagulation, fibrinolysis, and cytokine profile during laparoscopic and open cholecystectomy. Surg Endosc. 2004;18:1090–1096. doi: 10.1007/s00464-003-8819-0. [DOI] [PubMed] [Google Scholar]
- 36.Deitch EA. Multiple organ failure. Pathophysiology and potential future therapy. Ann Surg. 1992;216:117–134. doi: 10.1097/00000658-199208000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aydin C, Teke Z, Aytekin F, Yenisey C, Kabay B, Simsek NG, Tekin K. Tempol prevents harmful effects of remote ischemia reperfusion injury on healing of experimental colonic anastomoses. Int J Colorectal Dis. 2007;22:325–331. doi: 10.1007/s00384-006-0149-y. [DOI] [PubMed] [Google Scholar]
- 38.Ng CS, Wan S, Arifi AA, Yim AP. Inflammatory response to pulmonary ischemia-reperfusion injury. Surg Today. 2006;36:205–214. doi: 10.1007/s00595-005-3124-2. [DOI] [PubMed] [Google Scholar]
- 39.Dodds WJ, Dent J, Hogan WJ, Helm JF, Hauser R, Patel GK, Egide MS. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982;307:1547–1552. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- 40.Holloway RH, Dent J. Pathophysiology of gastroesophageal reflux. Lower esophageal sphincter dysfunction in gastroesophageal reflux disease. Gastroenterol Clin North Am. 1990;19:517–535. [PubMed] [Google Scholar]
- 41.Phillips SF, Quigley EM, Kumar D, Kamath PS. Motility of the ileocolonic junction. Gut. 1988;29:390–406. doi: 10.1136/gut.29.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malbert CH. The ileocolonic sphincter. Neurogastroenterol Motil. 2005;17 Suppl 1:41–49. doi: 10.1111/j.1365-2982.2005.00657.x. [DOI] [PubMed] [Google Scholar]


