Table 1.
Optimization of the redox-neutral amination reaction.a
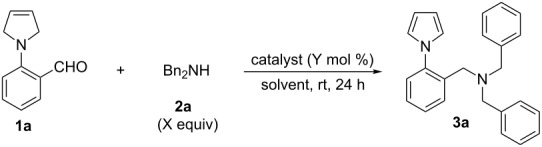 | |||||
| entry | catalyst | X | Y | solvent | yield (%)b |
| 1 | PhCOOH | 1.5 | 10 | DCE | 50 |
| 2 | CF3COOH | 1.5 | 10 | DCE | 87 |
| 3 | p-TsOH.H2O | 1.5 | 10 | DCE | 90 |
| 4 | Sc(OTf)3 | 1.5 | 10 | DCE | 94 |
| 5 | Cu(OTf)2 | 1.5 | 10 | DCE | 94 |
| 6 | Zn(OTf)2 | 1.5 | 10 | DCE | 97 |
| 7 | AlCl3 | 1.5 | 10 | DCE | 76 |
| 8 | ZnCl2 | 1.5 | 10 | DCE | 95 |
| 9 | ZnCl2 | 1.5 | 10 | CH2Cl2 | 97 |
| 10 | ZnCl2 | 1.5 | 10 | CHCl3 | 95 |
| 11 | ZnCl2 | 1.5 | 10 | toluene | 94 |
| 12 | ZnCl2 | 1.5 | 10 | CH3CN | 96 |
| 13 | ZnCl2 | 1.5 | 10 | THF | 71 |
| 14 | ZnCl2 | 1.2 | 10 | CH2Cl2 | 97 |
| 15 | ZnCl2 | 1.0 | 10 | CH2Cl2 | 93 |
| 16 | ZnCl2 | 1.2 | 5 | CH2Cl2 | 95 |
| 17 | ZnCl2 | 1.2 | 2 | CH2Cl2 | 91 |
a1a (0.5 mmol), 2a (X equiv), catalyst (Y mol %), solvent (5 mL), room temperature, 24 h. bIsolated yield.
