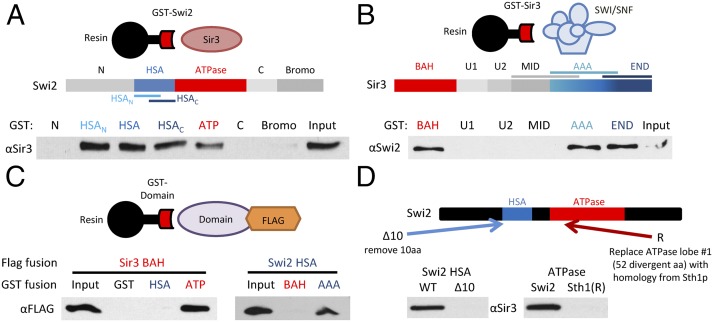
Fig. 2.
Swi2p and Sir3p have multiple interaction domains. (A) Schematic shows Swi2p domains. GST–Swi2 fusion proteins were used in pull-down assays with full-length Sir3p. GST-bound fractions were analyzed by Western blot. Shown is 10% of Input. (B) Schematic shows Sir3p domains. GST–Sir3 fusions were used in pull-down studies with the SWI/SNF complex. Bound fractions were assayed by Western blot as in A. (C) GST–Swi2 or GST–Sir3 fusion proteins were incubated with FLAG-tagged Swi2p or Sir3p domains, and interactions were identified by GST pull-down and Western analyses. (D) Swi2p alterations that disrupt Sir3p interactions. Schematic depicts alterations within either the Swi2p HSA or ATPase domain. The Δ10 derivative removes Swi2p residues 613–623; the Sth1(R) derivative replaces Swi2p residues 836–885 with the homologous region from Sth1 (residues 539–588). GST–Swi2 fusions harboring the indicated alterations were used in GST pull-downs with full-length Sir3p. Note that these binding assays used the individual HSA and ATPase regions of Swi2p.