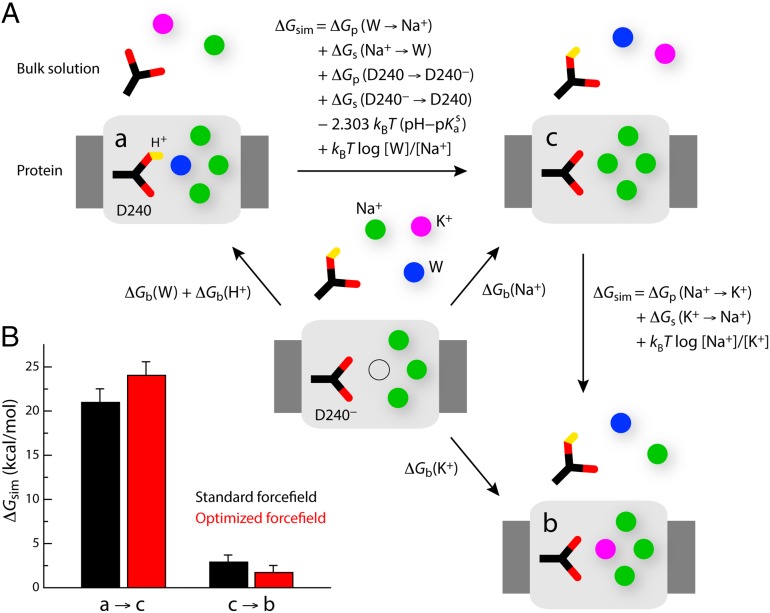
Fig. 3.
Thermodynamic analysis of the three ion occupancy configurations with best structural consistency with the NCX_Mj crystal structure. (A) Thermodynamic cycles used to deduce the alchemical transformations (ΔGsim) that are required to evaluate the relative free energies of the three ion configurations considered. (B) Calculated free-energy differences between configurations a and c, and between configurations c and b. The sum of these is the free-energy difference between a and b. It was assumed that pH = 7, [Na+] = [K+] = 100 mM, [W] = 55 M, and pKas = 3.86. The last two terms in the expression of the free-energy difference between a and c are thus -4.28 and 3.73 kcal/mol, respectively; and the last term in the expression of the free-energy difference between c and b is zero. Note that the volumes occupied by Na+, K+, or water at the Smid site are approximately equivalent, and thus the related entropic contributions are omitted. Free-energy values are reported for the standard CHARMM27 force field, as well as for a modified version in which the Lennard–Jones Rmin parameter for the Na+–carboxylate and K+–carboxylate interactions are optimized so as to reproduce osmotic pressure and binding affinity measurements (SI Methods and Figs. S3 and S4). The values shown are means of two independent free-energy calculations carried out in opposite directions, e.g., a to c and c to a. The differences between the calculated values in each direction are shown as error bars.