Fig. 5.
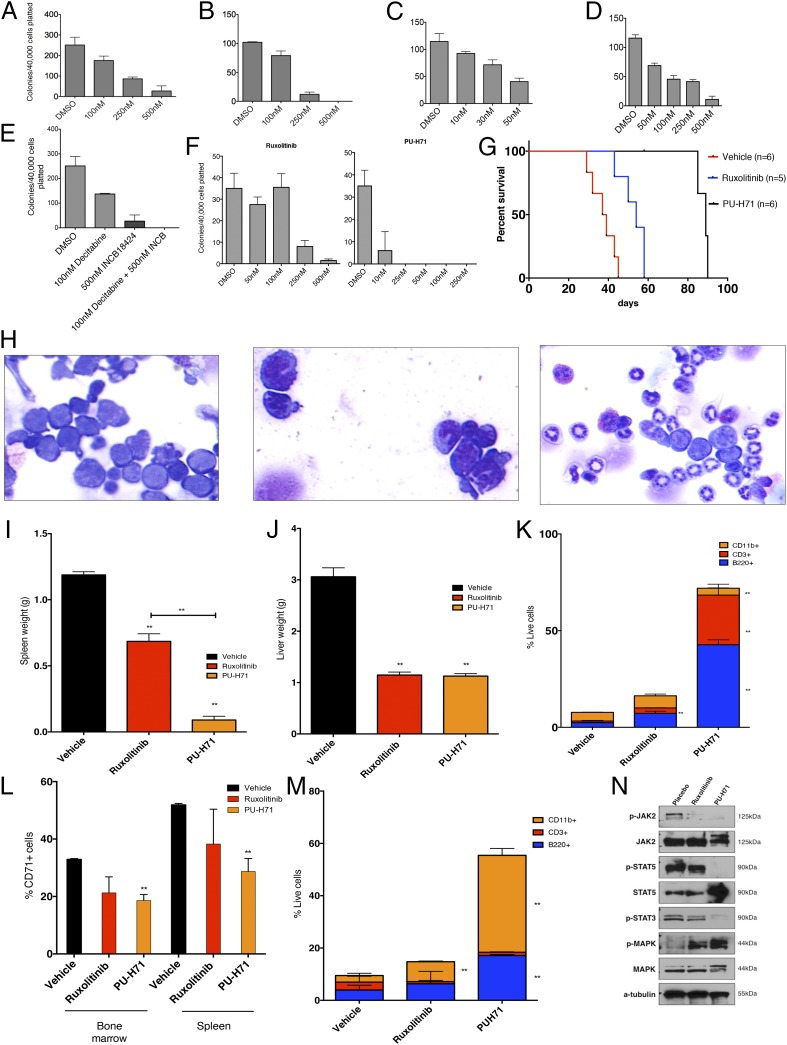
Pharmacologic inhibition of JAK2 as well as degradation of JAK2 alone and in combination with other therapies inhibits Tp53-KO/JAK2V617F leukemia. (A–E) The clonogenic capacity of Tp53-KO/JAK2V617F leukemic bone marrow cells in methylcellulose is inhibited on exposure to the JAK1/2 inhibitor ruxolitinib (A), the JAK1/2 inhibitor CTY387 (B), the Hsp90 inhibitor PU-H71 (C), decitabine (D), and a combination of decitabine and ruxolitinib (INCB18424) (E). For each condition, 40,000 spleen-derived cells were plated. (F) Clonogenic capacity of TP53 mutant/JAK2V617F human leukemic bone marrow cells in methylcellulose is inhibited on exposure to either ruxolitinib or PU-H71. (G) Treatment with either ruxolitinib or PU-H71 significantly prolongs survival (P < 0.01, log-rank test) of Tp53-KO/JAK2V617F leukemic mice relative to vehicle, and treatment with PU-H71 significantly prolongs survival compared with ruxolitinib (P < 0.01, log-rank test). PU-H71 was discontinued 1 wk after all ruxolitinib-treated mice were deceased. (H) Bone marrow cytospins demonstrating a predominance of blasts in vehicle-treated mice (Left), compared with increasing evidence of granulocytic maturation in mice treated with ruxolitinib (Center) and PU-H71 (Right). (I and J) Treatment with ruxolitinib or PU-H71 results in significant reductions in spleen (I; P < 0.05, P < 0.01, respectively) and liver (J; P < 0.01 for both treatments relative to vehicle) weights. PU-H71 significantly reduced spleen weight compared with ruxolitinib as well (P < 0.01, t test). (K) Treatment with ruxolitinib results in expansion of CD3+ cells (P < 0.05, t test), and treatment with PU-H71 results in expansion of CD3+ (P < 0.05, t test), CD11b++ (P < 0.01, t test), and B220+ (P < 0.01, t test) populations in spleens of treated mice. (L) The proportion of CD71+ cells is reduced in bone marrow of ruxolitinib- and PU-H71–treated mice (P < 0.05, t test, for PU-H71) and in spleen of ruxolitinib- and PU-H71–treated mice (P < 0.05, t test, for PU-H71). (M) Treatment with ruxolitinib or PU-H71 results in the expansion of CD11b+ (P < 0.05 for ruxolitinib, and P < 0.01 for PU-H71, t test) and B220+ (P < 0.01 for PU-H71, t test) cells in bone marrow of treated mice. (N) Western blot demonstrating reduction in phospho-JAK2 and phospho-STAT5 levels in mice treated with ruxolitinib or PU-H71 relative to placebo. **P < 0.05.