Fig. 4.
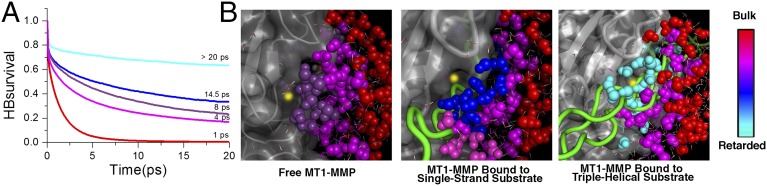
Effect of substrates on the gradients of coupled water motions. (A) Changes in hydration dynamics on Michaelis complex formation. Shown are the averaged hydrogen bond lifetimes τHB of bulk water (red line; distance to closest protein atom > 10 Å), water molecules solvating MT1-MMP (magenta line; distance to closest enzyme atom < 3 Å), and water solvating the zinc ion (distance to catalytic zinc ion < 6 Å) in the unbound MMP–triple-helical substrate system (purple line), the MMP–single-stranded substrate system (blue line), and the MMP–triple-helical substrate system (cyan line). (B) A gradient of water motions [from retarded water molecules (cyan) to bulk (red)] is detected in the unbound MT1-MMP when the substrates are at a distance d of ∼7 Å from the zinc ion. When the substrates approach closer to the zinc ion (d ∼ 4.9 Å), an additional decrease in the water HB lifetime at the active site for both substrates is observed. The gradient of water motions depends on the substrate: τHB is steeper when the MT1-MMP is bound to the triple-helical substrate (Right) than when it is bound to the single-stranded substrate (Center).
