Figure 1.
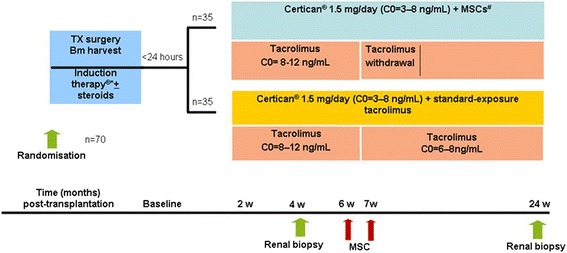
Study scheme. In total 70 patients will be included in the study, 18–75 years old. Patients will be randomised prior to transplantation. Thirty five of these patients will be included in the Certican®/MSC group and 35 patients in the Certican®/standard dose tacrolimus group. All patients will receive steroids (100 mg at day 1 to 3, 50 mg at day 4, 20 mg at day 5 to 15, 15 mg at day 15 to 21, and 10 mg after day 22) and induction treatment with alemtuzumab at day 0 and 1 (15 mg subcutaneously)*. Certican® dose will be 1.5 mg b.i.d. with trough levels between 3 and 8 ng/ml. Tacrolimus will be started orally 3 h before surgery (initial dose 2x5 mg ). In the first 6 weeks target trough levels are aimed at 10 ng/ml (range 8 to 12 ng/ml) for tacrolimus and thereafter 6–8 ng/ml. In the MSC treated group, BM will be harvested just prior to the renal transplantation and MSCs will be cultured in the GMP laboratory. Patients will receive 2 doses of a target of 1,5x106 autologous BM MSCs per/kg body weight IV (range 1-2x106) 7 days apart, 6 and 7 weeks after transplantation. The dose of tacrolimus will be reduced to 50% at the time of the second MSC infusion and completely withdrawn 1 week later. Patients will receive at that time point 15 mg of prednisolone. In all patients a renal biopsy will be performed at 4 weeks and at 6 months and scored according to the Banff criteria.
