Abstract
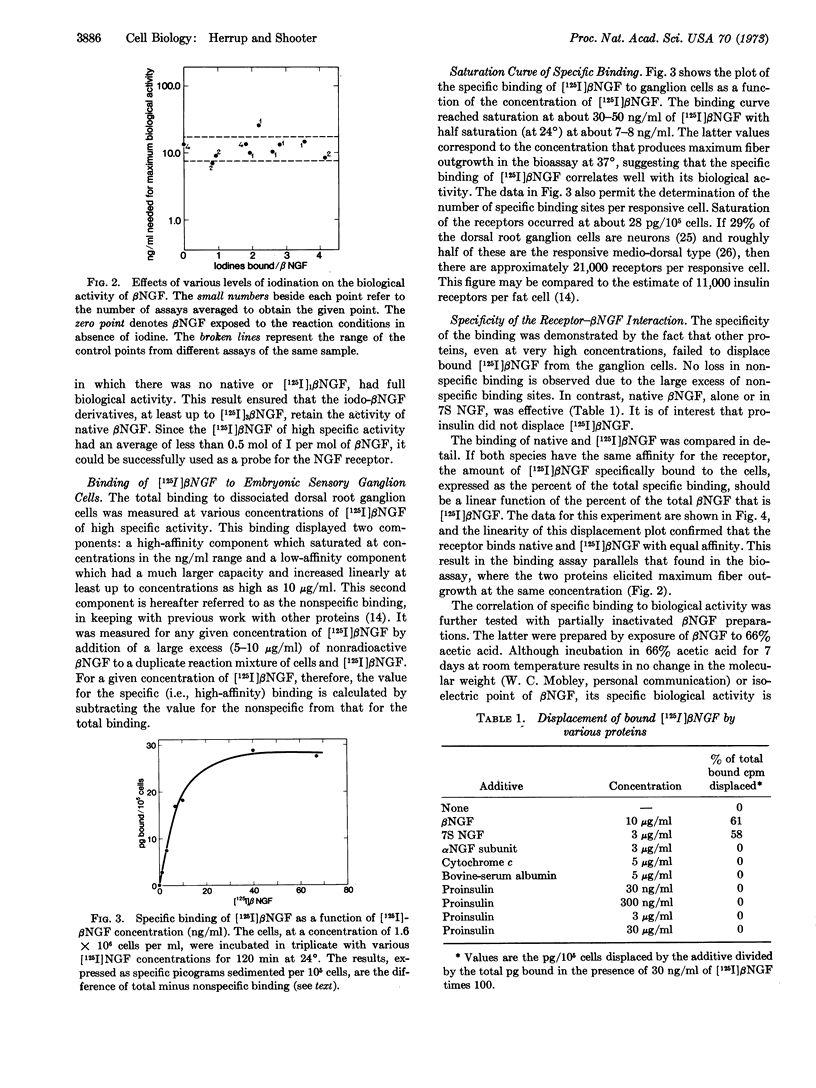
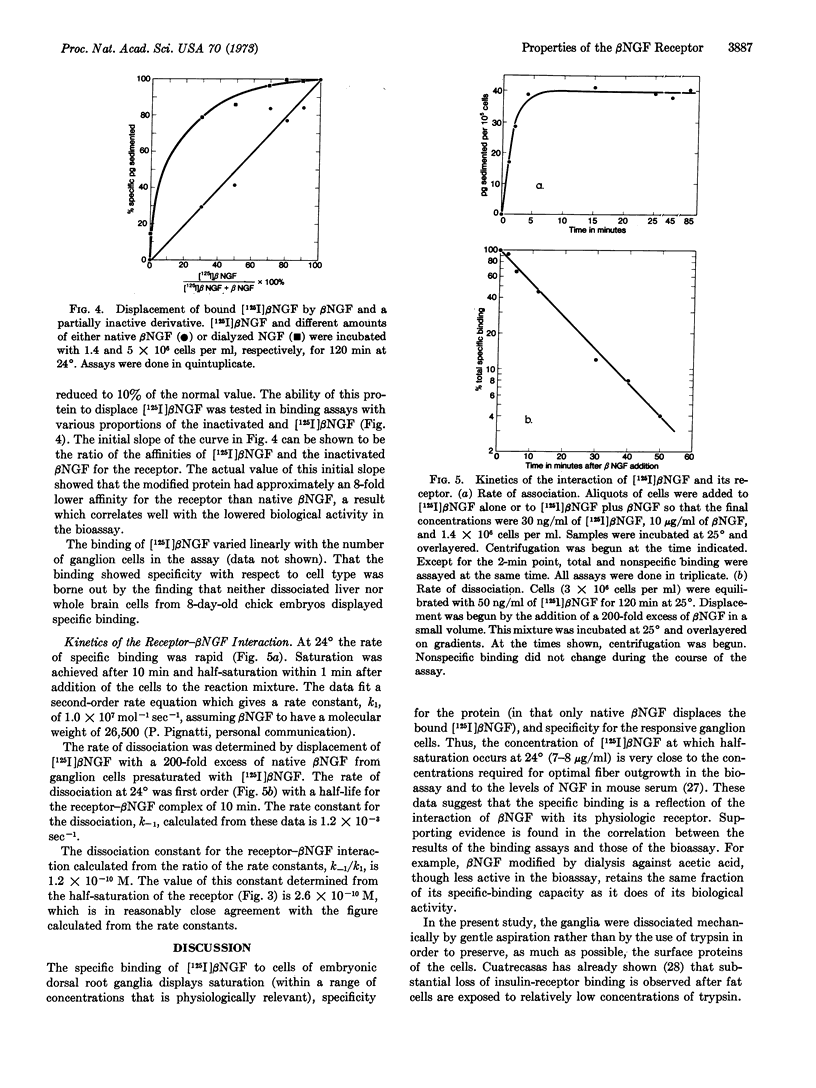
Iodination of the β nerve growth factor protein up to 4.1 iodines per mole of nerve growth factor was achieved with no loss of biological activity. At this level of incorporation no native or monoiodo nerve growth factor remained, the major species being the tri- and tetraiodo derivatives. An 125I-labeled β nerve growth factor of high specific activity containing 0.5 iodine per mole of β nerve growth factor was used to determine the specific binding of nerve growth factor to dorsal root ganglion cells of 8-day-old embryonic chicks. The specific binding of 125I-labeled nerve growth factor reached saturation at 30-50 ng/ml and half-saturation at 7-8 ng/ml (0.26 nM). The number of receptors per responsive medio-dorsal cell was calculated to be about 2 × 104. The pattern of displacement of bound 125I-labeled β nerve growth factor by native β nerve growth factor showed that the two proteins had identical affinities for the receptor. The specificity of the binding for β nerve growth factor was demonstrated by the fact that only native β nerve growth factor displaced the bound 125I-labeled form. Partially inactivated derivatives of β nerve growth factor retained the same fraction of their specific-binding capacity as of their biological activity. The specificity of the binding for cell type was shown by the lack of any specific component of 125I-labeled β nerve growth factor binding to liver or brain cells. The rate constant for association of β nerve growth factor with its receptor, k1, was 1.0 × 107 mol-1 sec-1 and the rate constant for dissociation, k-1, 1.2 × 10-8 sec-1.
Keywords: iodination, membrane
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti R. H., Bradshaw R. A. Nerve growth factor from mouse submaxillary gland: amino acid sequence. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2417–2420. doi: 10.1073/pnas.68.10.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeletti R. H., Mercanti D., Bradshaw R. A. Amino acid sequences of mouse 2.5S nerve growth factor. I. Isolation and characterization of the soluble tryptic and chymotryptic peptides. Biochemistry. 1973 Jan 2;12(1):90–100. doi: 10.1021/bi00725a017. [DOI] [PubMed] [Google Scholar]
- BORNSTEIN M. B. Reconstituted rattail collagen used as substrate for tissue cultures on coverslips in Maximow slides and roller tubes. Lab Invest. 1958 Mar-Apr;7(2):134–137. [PubMed] [Google Scholar]
- Burnham P., Raiborn C., Varon S. Replacement of nerve-growth factor by ganglionic non-neuronal cells for the survival in vitro of dissociated ganglionic neurons. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3556–3560. doi: 10.1073/pnas.69.12.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COHEN S. Purification and metabolic effects of a nerve growth-promoting protein from snake venom. J Biol Chem. 1959 May;234(5):1129–1137. [PubMed] [Google Scholar]
- Cohen S., Levi-Montalcini R. A NERVE GROWTH-STIMULATING FACTOR ISOLATED FROM SNAKE VENOM. Proc Natl Acad Sci U S A. 1956 Sep;42(9):571–574. doi: 10.1073/pnas.42.9.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. PURIFICATION OF A NERVE-GROWTH PROMOTING PROTEIN FROM THE MOUSE SALIVARY GLAND AND ITS NEURO-CYTOTOXIC ANTISERUM. Proc Natl Acad Sci U S A. 1960 Mar;46(3):302–311. doi: 10.1073/pnas.46.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin--receptor interactions in adipose tissue cells: direct measurement and properties. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1264–1268. doi: 10.1073/pnas.68.6.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrecasas P. Perturbation of the insulin receptor of isolated fat cells with proteolytic enzymes. Direct measurement of insulin-receptor interactions. J Biol Chem. 1971 Nov;246(21):6522–6531. [PubMed] [Google Scholar]
- EHRMANN R. L., GEY G. O. The growth of cells on a transparent gel of reconstituted rat-tail collagen. J Natl Cancer Inst. 1956 Jun;16(6):1375–1403. [PubMed] [Google Scholar]
- Frazier W. A., Angeletti R. H., Bradshaw R. A. Nerve growth factor and insulin. Science. 1972 May 5;176(4034):482–488. doi: 10.1126/science.176.4034.482. [DOI] [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Hendry I. A. Developmental changes in tissue and plasma concentrations of the biologically active species of nerve growth factor in the mouse, by using a two-site radioimmunoassay. Biochem J. 1972 Aug;128(5):1265–1272. doi: 10.1042/bj1281265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hier D. B., Arnason B. G., Young M. Studies on the mechanism of action of nerve growth factor. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2268–2272. doi: 10.1073/pnas.69.8.2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI-MONTALCINI R., MEYER H., HAMBURGER V. In vitro experiments on the effects of mouse sarcomas 180 and 37 on the spinal and sympathetic ganglia of the chick embryo. Cancer Res. 1954 Jan;14(1):49–57. [PubMed] [Google Scholar]
- Levi-Montalcini R., Angeletti P. U. Nerve growth factor. Physiol Rev. 1968 Jul;48(3):534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R., Booker B. DESTRUCTION OF THE SYMPATHETIC GANGLIA IN MAMMALS BY AN ANTISERUM TO A NERVE-GROWTH PROTEIN. Proc Natl Acad Sci U S A. 1960 Mar;46(3):384–391. doi: 10.1073/pnas.46.3.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J. An enzymic method for the trace iodination of immunoglobulins and other proteins. Biochem J. 1969 Jun;113(2):299–305. doi: 10.1042/bj1130299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partlow L. M., Larrabee M. G. Effects of a nerve-growth factor, embryo age and metabolic inhibitors on growth of fibres and on synthesis of ribonucleic acid and protein in embryonic sympathetic ganglia. J Neurochem. 1971 Nov;18(11):2101–2118. doi: 10.1111/j.1471-4159.1971.tb05069.x. [DOI] [PubMed] [Google Scholar]
- Roisen F. J., Murphy R. A., Pichichero M. E., Braden W. G. Cyclic adenosine monophosphate stimulation of axonal elongation. Science. 1972 Jan 7;175(4017):73–74. doi: 10.1126/science.175.4017.73. [DOI] [PubMed] [Google Scholar]
- Smith A. P., Varon S., Shooter E. M. Multiple forms of the nerve growth factor protein and its subunits. Biochemistry. 1968 Sep;7(9):3259–3268. doi: 10.1021/bi00849a032. [DOI] [PubMed] [Google Scholar]
- Varon S., Nomura J., Shooter E. M. The isolation of the mouse nerve growth factor protein in a high molecular weight form. Biochemistry. 1967 Jul;6(7):2202–2209. doi: 10.1021/bi00859a043. [DOI] [PubMed] [Google Scholar]
- Yamada K. M., Wessells N. K. Axon elongation. Effect of nerve growth factor on microtubule protein. Exp Cell Res. 1971 Jun;66(2):346–352. doi: 10.1016/0014-4827(71)90687-2. [DOI] [PubMed] [Google Scholar]