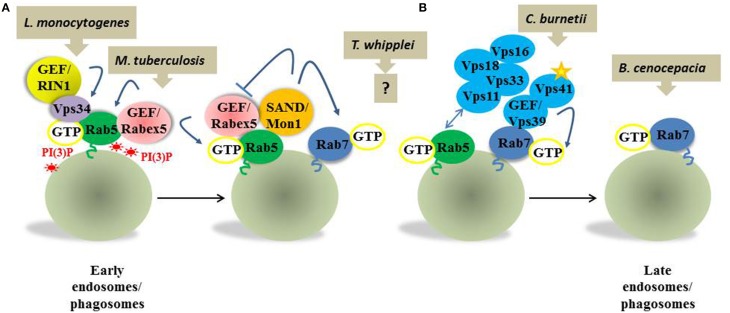
Figure 1.
Simplified view of the molecular mechanisms involved in Rab5 to Rab7 transition exploited by bacterial pathogens. Rab5 in its active form recruits on the early compartment a GEF Rabex5, which stabilizes Rab5 recruitments to the membrane, and Vps34, the PI(3)P kinase that regenerates PI(3)P. Rab5 activity at the early endosomes/phagosomes is also regulated by GEF RIN1. Two events take place on the early endosomes, which implicate simultaneous recruitment of Rab7 and maturation toward the late endosomes/phagosomes. (A) SAND1/Mon1 binds Rabex5 and displaces it from early endosome, inactivating the Rab5 recruitment loop. Additionally, SAND1/Mon1 interacts with a Rab7 GEF, the Vps39 subunit of the HOPS complex (blue). (B) The HOPS complex Vps11 subunit interacts with Rab5-GTP, probably stabilizing the Rab5-Rab7 transition. Interestingly p38α-MAPK dependent phosphorylation of the HOPS complex Vps41 subunit also seems important for Rab7 recruitment. Upon Rab7 recruitment and activation, Rab5 is released and early endosomes/phagosomes mature in late endosomes/phagosomes. For each of the described steps a distinct subversion mechanism has been evolved by bacterial pathogens. L. monocytogenes engages RIN1 to promote accumulation in a Rab5-positive compartment. M. tuberculosis inactivates Vps34 and consumes PI(3)P, interfering with Rab5 recruitment. T. whipplei blocks the transition in a Rab5- and Rab7 positive state by an unknown mechanism. C. burnetii interferes with Vps41 phosphorylation and Rab7 recruitment. B. cenocepacia affects Rab7 activation on the membranes.