Figure 3.
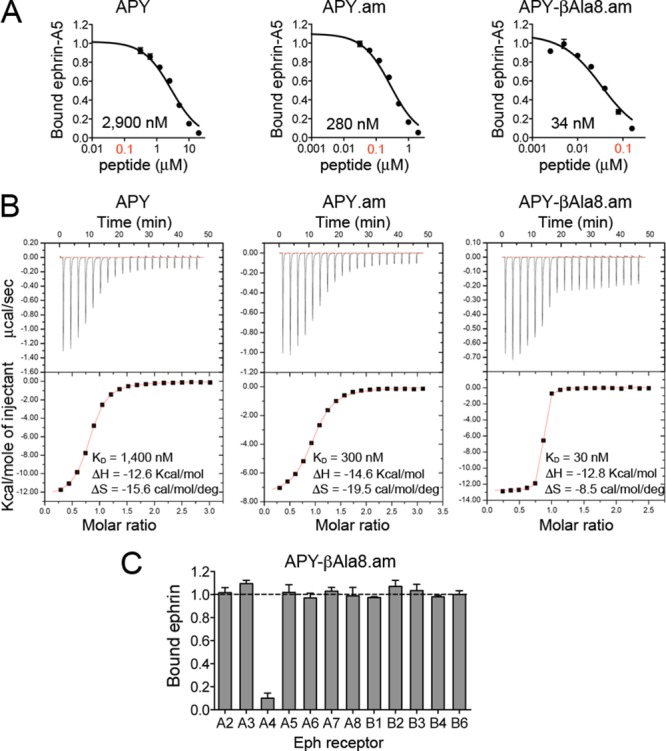
C-terminal amidation of APY and replacement of Gly8 with βAla yield the nanomolar EphA4 antagonist APY-βAla8.am. (A) Representative curves showing inhibition of ephrin-A5 AP binding to immobilized EphA4 Fc by APY derivatives in ELISAs. Bound ephrin-A5 values were normalized to those for bound ephrin-A5 in the absence of peptide and averages from triplicate measurements ± SE are shown. IC50 values are shown under each curve, and the 0.1 μM peptide concentration is in red font. (B) Isothermal titration calorimetry profiles for peptide binding to EphA4 (upper part of each panel) and plots of the integrated values for the reaction heats (after blank subtraction and normalization to the amount of injected peptide) versus EphA4/peptide molar ratio (lower part of each panel). (C) Eph receptor selectivity for APY-βAla8.am. ELISA measuring inhibition of ephrin-A5 AP binding to immobilized EphA Fc receptors and ephrin-B2 AP binding to EphB Fc receptors shows that 3.7 μM APY-βAla8.am selectively inhibits ephrin binding to EphA4. Bound ephrin is the signal in the presence of APY-βAla8.am normalized to the signal without peptide. Averages from triplicate measurements ± SE are shown.
