Figure 4.
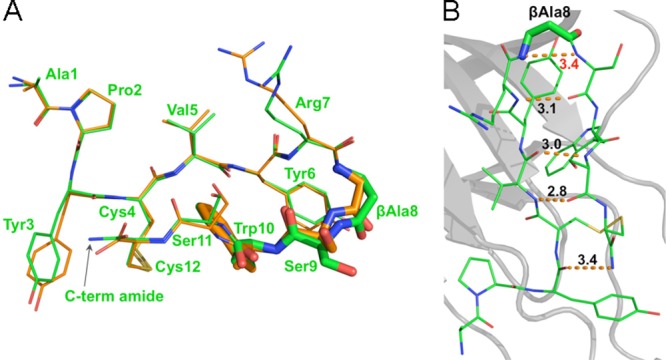
Crystal structure of APY-βAla8.am bound to EphA4 reveals critical differences from APY. (A) Overlay of APY-βAla8.am (green) and APY (orange) shows marked differences in the β-turn region, particular in the βAla8, Ser9, and Trp10 residues (highlighted by stick representation). Residues are labeled for the APY-βAla8.am peptide. (B) Intramolecular hydrogen bonds of APY-βAla8.am. Overall intramolecular hydrogen bond patterns and conformations are more favorable for APY-βAla8.am than for APY peptide, including the presence of an additional hydrogen bond between the C-terminal amide and Tyr3 of APY-βAla8.am. Molecule A is shown for both peptides, whereas all four molecules in the asymmetric unit are shown in Supporting Information Figure 3.
