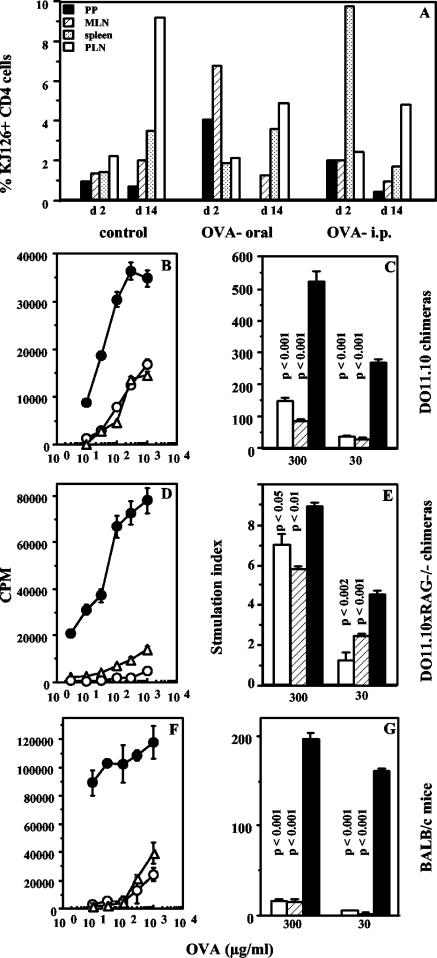
FIG. 6.
Soluble OVA given orally or systemically has similar effects in vivo. (A) Expansion of antigen-specific cells in vivo following oral or i.p. OVA. Proportions of KJ1-26+ CD4 cells in PP, MLN, spleens, and PLN of BALB/c mice chimeric for DO11.10 cells given 5 mg of OVA orally or 1 mg or OVA i.p. or left untreated (control), 2 days after oral or i.p treatment (d2) or 1 week after s.c. immunization of treated and untreated mice (d14), in an oral tolerance experiment are shown. Data are representative of those from two experiments with two mice per group per time point. (B to G) Induction of systemic T-cell hyporesponsiveness by oral or i.p. administration of OVA. Proliferation of PLN cells from BALB/c mice chimeric for DO11.10 cells (B and C), from mice chimeric for DO11.10 × RAG−/− cells (D and E), and from normal BALB/c mice (F and G) 1 week after s.c. immunization with OVA in CFA is shown. Filled circles and bars, mice untreated before s.c immunization; open circles and bars, mice given 5 mg of OVA orally 6 days earlier; triangles and hatched bars, mice given 1 mg of OVA i.p. 6 days earlier. Data are represented as counts per minute in response to titrating doses of OVA in vitro (P < 0.05 [B], P < 0.004 [D], and P < 0.005 [F]) and as the SI at two doses of OVA Data are representative of those from at least two experiments each, and P values for treated versus untreated groups are indicated above each bar.