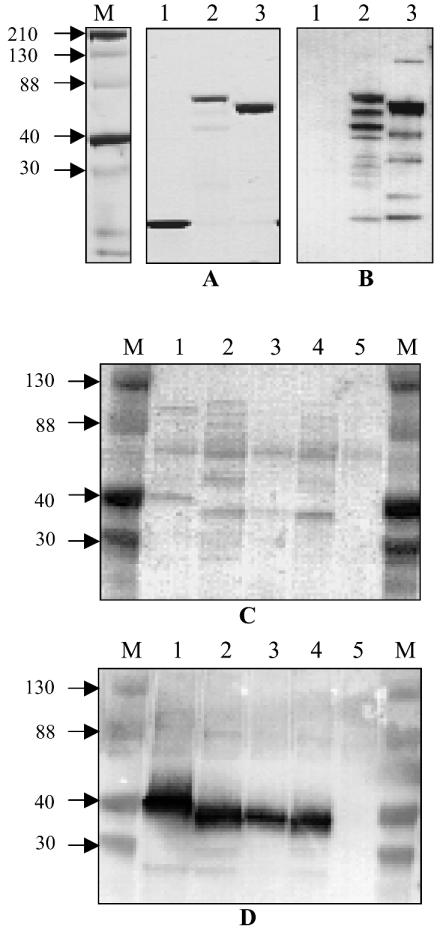
FIG. 1.
(A and B) Recombinant DRS expressed in E. coli as thioredoxin fusion proteins. The proteins were purified as described in the text. (A) Coomassie blue-stained SDS-polyacrylamide gels. (B) Western blot reacted with a mixture (1:1) of polyclonal antibodies against SIC and DRS. Lanes 1 to 3 show His-tagged thioredoxin, SIC (BSA5)-thioredoxin fusion protein, and DRS (NS488)-thioredoxin fusion protein, respectively. The antibodies did not react with thioredoxin. (C and D) Presence of DRS in culture supernatants of DRS-expressing strains. The culture supernatants were processed as described in the text. The SDS-polyacrylamide gels were transferred to a nitrocellulose membrane (Amersham) and either stained with Ponceau S (C) or reacted with a mixture of SIC and DRS antibodies (D) as described for panel B. Lanes 1 to 5 are culture supernatants from NS844, DRV1, NS488, NS25, and NS27, respectively. NS27 is a SIC- and DRS-negative control. Channels marked M contain Kaleidoscope (Bio-Rad) markers, and approximate sizes of the proteins are shown (in kilodaltons).