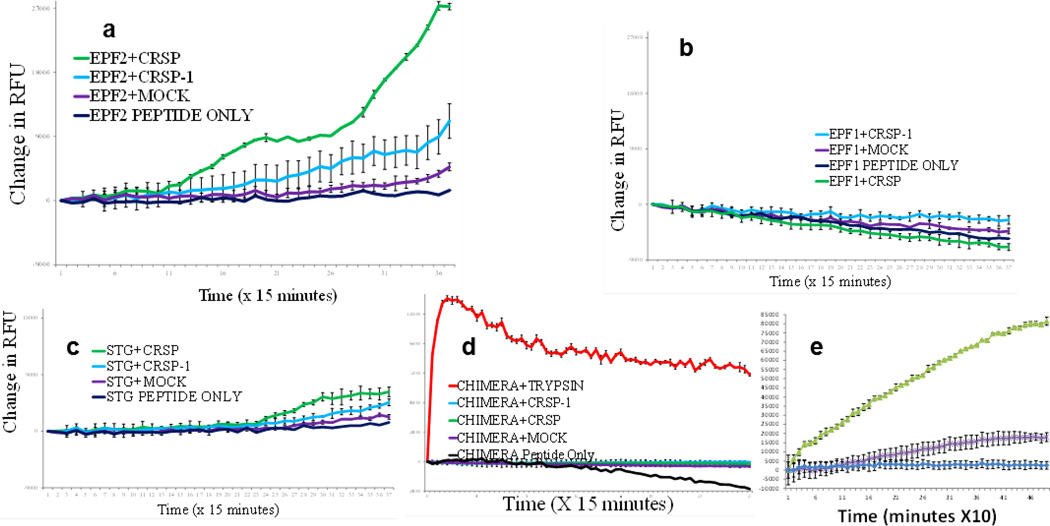
Extended Data Figure 6. CRSP cleaves synEPF2 in vitro.
In vitro cleavage reactions over time of synthetic EPF family peptides incubated with CRSP, (mutated) CRSP-1 and negative control (mock, wheat germ extract only) proteases. The synEPF peptides are flanked by fluorophore and quencher moieties, and fluorescence can be measured when the quencher–fluorophore interaction is disrupted by cleavage of the synEPF peptide. EPF2 (a); EPF1 (b); STOMAGEN (STG) (c); a chimaeric peptide of EPF2, including seven amino acid substitutions corresponding to STOMAGEN in the region of the cleavage (d). The EPF2 peptide that was used comprises the 69 carboxyterminal amino acids of the native EPF2 peptide and includes the predicted cleavage site. This peptide lacks the 51 amino-terminal amino acids of the native EPF2 peptide. We mapped an in vitro cleavage site of the synthetic EPF2 peptide using MS/MS analyses, and our results show predominant cleavage at the site in bold: SKNGGVEMEMYPTGSSLPD|CSYACGACSPC. When aligned with the STOMAGEN protein sequence, this in vitro cleavage site of EPF2 by CRSP is within seven residues of the native STOMAGEN peptide cleavage site23,27. It remains to be determined whether an EPF2 cleavage site corresponding to the STOMAGEN cleavage site23,27 occurs in vivo. The CHIMERA peptide was also cleaved by trypsin to demonstrate the functionality of the synthetic fluorogenic peptide (the EPF1 and STOMAGEN peptides also showed a robust fluorescence signal when cleaved with trypsin). To test the specificity of CRSP-mediated EPF2 cleavage, we conducted cleavage experiments with a re-designed EPF2–STOMAGEN chimaeric peptide. This peptide included 7 amino acid substitutions in the EPF2 sequence, converting a stretch of 12 EPF2 residues into the aligned STOMAGEN sequence (the 12 residue stretch spans the LPD|CS site). The modified EPF2 cleavage site containing the STOMAGEN sequence is SKNGGVEMEMYPIGSTA PTCTYNEGACSPC. We changed the D (in the LPD|CS site) to a T since this corresponds to the sequence of STOMAGEN and EPFL4, a negative regulatory peptide related to EPF2. The modified sequence contained the STOMAGEN-specific TTNE motif. These experiments show that CRSP mediated cleavage is abolished in this chimaeric EPF2–STOMAGEN peptide. Fluorescence data were normalized for background fluorescence by using buffer only controls, and the change in the relative fluorescence was calculated by subtracting the initial fluorescence measurement for each sample. e, The change in the relative fluorescence emitted over time on cleavage of the synthetic EPF2 peptide (synEPF2) by CRSP in the presence or absence of protease inhibitors is shown (Methods). In all panels n = 3. Error bars, mean ± s.e.m.