Abstract
Endoplasmic reticulum (ER) stress is implicated as a significant contributor to neurodegeneration and cognitive dysfunction. Previously, we reported that the widely used pyrethroid pesticide deltamethrin causes ER stress-mediated apoptosis in SK-N-AS neuroblastoma cells. Whether or not this occurs in vivo remains unknown. Here, we demonstrate that repeated deltamethrin exposure (3 mg/kg every 3 days for 60 days) causes hippocampal ER stress and learning deficits in adult mice. Repeated exposure to deltamethrin caused ER stress in the hippocampus as indicated by increased levels of C/EBP-homologous protein (131%) and glucose-regulated protein 78 (96%). This was accompanied by increased levels of caspase-12 (110%) and activated caspase-3 (50%). To determine whether these effects resulted in learning deficits, hippocampal-dependent learning was evaluated using the Morris water maze. Deltamethrin-treated animals exhibited profound deficits in the acquisition of learning. We also found that deltamethrin exposure resulted in decreased BrdU-positive cells (37%) in the dentate gyrus of the hippocampus, suggesting potential impairment of hippocampal neurogenesis. Collectively, these results demonstrate that repeated deltamethrin exposure leads to ER stress, apoptotic cell death in the hippocampus, and deficits in hippocampal precursor proliferation, which is associated with learning deficits.
Keywords: deltamethrin, ER stress, adult neurogenesis, water maze, hippocampus
Pyrethroid insecticides are one of the most widely used agricultural and household pesticides and their use has been increasing over recent years since the use of many organophosphates has been restricted by the United States Environmental Protection Agency (Morgan, 2012). Although exposure of the general population to pyrethroids is generally low, significant levels of pyrethroid metabolites, including those of deltamethrin, have been found in human urine and high levels of exposure have been observed in pesticide applicators (Aprea et al., 1997; Berkowitz et al., 2003; Calvert et al., 2003, Kimata et al., 2009; Trunnelle et al., 2014, Ueyama et al., 2009). In general, pyrethroid toxicity is thought to be low in humans because of efficient metabolic detoxication. Pyrethroids are rapidly hydrolyzed by serum carboxylesterases (Crow et al., 2007); however, human serum lacks carboxylesterase activity, suggesting humans may have a reduced capacity to metabolize pyrethroids. In addition, there are other species differences in deltamethrin metabolism based on hepatic P450s that may influence susceptibility (Godin et al., 2007). Most recently, a physiologically based pharmacokinetic study demonstrated that exposure to the pyrethroid deltamethrin was predicted to generate a 2-fold greater peak brain concentration in humans compared with rats (Godin et al., 2010). Taken together, these data suggest that humans may be at greater risk of the toxic effects of pyrethroids than previously thought.
Residual neurological symptoms, such as disruption of cognitive function, have been reported in individuals who are highly exposed to pyrethroids (Muller-Mohnssen, 1999). However, the mechanism by which this occurs is not clear. Higher level acute exposure to the type II pyrethroid deltamethrin causes apoptosis both in vivo (Wu and Liu, 2000) and in vitro (Elwan et al., 2006; Wu et al., 2003). Recent work from our laboratory identified that deltamethrin-induced apoptosis in vitro was through activation of the endoplasmic reticulum (ER) stress pathway (Hossain and Richardson, 2011). Persistent activation of the ER stress pathway is linked to progressive loss of neurons leading to neurodegeneration and cognitive dysfunction (Hotamisligil, 2010; Salminen et al., 2009), which may be the result of reduced synaptic plasticity and neuronal viability in the hippocampus (Sama and Norris, 2013). To date, there are no data regarding the ability of pyrethroids to produce ER Stress in vivo and whether this would result in neurobehavioral consequences.
Here, we report that repeated deltamethrin exposure elicits hippocampal ER stress and learning deficits in adult mice. Further, these effects were associated with decreased proliferation of cells in the dentate gyrus (DG) of the hippocampus, suggesting a potential effect on adult hippocampal neurogenesis. As these effects occurred at a dose (3 mg/kg) near the lowest observed adverse effect level (LOAEL) of 2.5 mg/kg established by the EPA (2010) in risk assessment of deltamethrin, these findings underscore the need for additional research to determine whether hippocampal neurogenesis is affected and whether or not these effects might occur in the human population.
MATERIALS AND METHODS
Animals
Male C57BL/6 mice, 10 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME) and housed 5 per cage on a 12-h light-dark cycle with food and water available ad libitum. Animal handling and experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the animal care committee of Rutgers-Robert Wood Johnson Medical School.
Treatment
Deltamethrin (purity 99%, Chem Service Inc., West Chester, PA) was dissolved in corn oil and administered at 0 (n = 17) or 3 mg/kg (n = 17) to mice via oral gavage every 3 days for 60 days. The dose of deltamethrin used in this study is near the LOAEL of 2.5 mg/kg/day derived from subchronic rat, subchronic dog, and chronic dog studies (Environmental Protection Agency (EPA), 2010) and did not cause any signs of toxicity such as tremor, salivation, ataxia, or decreased body weight gain throughout the experiment. This dosing paradigm was chosen to represent a subchronic exposure, while allowing animals 3 days for clearance of deltamethrin from the body. This was based on recent physiologically based pharmacokinetic (PBPK) study demonstrating that deltamethrin is rapidly metabolized and almost completely eliminated within 48 h of an oral administration at a dose of 3 mg/kg (Godin et al., 2010). At the end of the dosing paradigm, the 34 mice (n = 17 control and 17 deltamethrin) were randomly divided into 4 groups for behavioral (n = 6 per group), biochemical (n = 5 per group), immunohistochemical (n = 3 per group) or neurogenesis (n = 3 per group) studies. Three days after the last dose of deltamethrin, animals for biochemical analysis were euthanized with CO2 and hippocampi were dissected out, frozen on dry ice and stored at −80°C until processing for Western blots.
Western blot analysis
Western blots were performed as described previously (Hossain and Richardson, 2011). Hippocampal tissues were homogenized in tissue homogenization buffer (0.32 M sucrose, 5 mM HEPES; pH 7.4) supplemented with 0.1% protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Samples were centrifuged at 3500 rpm for 5 min at 4°C. Supernatants were collected and re-centrifuged at 14 000 rpm for 45 min at 4°C. Resulting pellets were re-suspended in 100 μl of homogenization buffer supplemented with 0.1% protease inhibitor. Protein concentrations were quantified using the bicinchoninic acid (BCA) assay (Smith et al., 1985), and 20 µg of protein sample was loaded per lane on 4–12% NuPAGE® Novex® Bis-Tris Mini Gels (Invitrogen, Carlsbad, CA). After electrophoresis, proteins from the gels were transferred to polyvinylidene difluoride (PVDF) membranes (Invitrogen, Carlsbad, CA). The membranes were incubated in 7.5% non-fat milk in 0.1% Tween-20 containing Tris buffered saline (TTBS) for 1 h at room temperature to block non-specific protein binding sites. The membranes were then incubated overnight at 4°C with anti-caspase-3 (1:500; cat # 9661, Cell Signaling, Danvers, MA), caspase-12 (1:1000; cat # 2202, Cell Signaling, Danvers, MA), anti-GRP78 (glucose-regulated protein 78) (1:1000; cat # 3177, Cell Signaling) and anti-CHOP (C/EBP-homologous protein) (1:500, cat # sc 575; Santa Cruz, CA) primary antibodies. After being washed 3 times with TTBS, the membranes were incubated with appropriate horseradish peroxidase-conjugated secondary antibodies for 1 h at room temperature. The specific antibody-bound protein was detected by SuperSignal® West Dura Extended Duration Substrate (Thermo Scientific, Rockford, IL) using Alpha Innotech Fluorochem (San Leandro, CA) imaging system and stored as a digital image. Membranes were then stripped for 15 min at room temperature with Pierce Stripping Buffer (Thermo Scientific, Rockford, IL) and re-probed with a monoclonal α-tubulin antibody to confirm equal protein loading.
Immunohistochemistry
Three days after the final deltamethrin dose, mice were anesthetized with sodium pentobarbital (50 mg/kg) and transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde in PBS (pH 7.4). Brains were removed and post-fixed in 4% paraformaldehyde at 4°C for 24 h, and then transferred into 30% sucrose with 0.1% sodium azide in PBS. Coronal sections through the entire hippocampus were cut (30 μm) on a freezing, sliding microtome and stored at −20°C in cryoprotectant solution (25% sucrose + 25% ethylene glycol in PBS).
Caspase-3 immunohistochemistry was performed on free-floating sections as described previously (Falluel-Morel et al., 2007). Briefly, every sixth section was taken for immunohistochemical analysis and each section was 180 µm apart. Sections were washed in PBS to remove cryoprotectant; and heat-induced antigen retrieval was performed by steaming sections in 0.1 M citrate buffer (pH 6.0) for 10 min at 97°C. Sections were then incubated with 0.3% hydrogen peroxide in methanol for 10 min to inactivate endogenous peroxidases. Sections were rinsed, blocked in PBS containing 10% normal goat serum and 0.3% Triton X-100 for 1 h and then incubated with rabbit monoclonal anti-caspase-3 (1:250; cat # 9661, Cell Signaling) in PBS containing 2% normal goat serum and 0.3% Triton X-100 overnight at 4°C. After rinsing 3 times with PBS, sections were incubated with biotinylated horse anti-mouse or goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) secondary antibody for 1 h at room temperature, followed by incubation in avidin–biotin peroxidase complex (ABC kit; Vector Laboratories) for 1 h at room temperature for amplification. Diaminobenzidine (DAB) fast-tab solution (Sigma, St. Louis, MO) was applied as a chromogen to localize the peroxidase. Sections were then rinsed in PBS, mounted onto slides (VWR, West Chester, PA) and counterstained with 0.25% Toluidine Blue (Sigma, St. Louis, MO).
Caspase-3 positive cell visualization and quantification
Following immunohistochemistry, dark brown caspase-3 positive cells from every sixth coronal section containing hippocampus were visualized using a Carl Zeiss Axiophot El- Einsatz microscope (Zeiss Inc., Germany) with a ProgRes® C14plus camera (Jenoptik Optical Systems GmbH) and ProgRes®CapturePro 2.8 software (Jenoptik Inc., Easthampton, MA). The positive cells from bilateral hippocampi were manually counted at higher magnification (×40) from a total of 6 sections per animal and 3 animals per group. The mean for each animal (from the 6 sections) then was averaged to obtain the group mean, and compared by an unpaired t-test with Welch’s correction.
Morris water maze
Three days after withdrawal of deltamethrin, mice (n = 6 per group) underwent Morris water maze training (Morris, 1984). Data were acquired and analyzed with a live video tracking system (Ethovision, Noldus). The maze consisted of a circular pool (100 cm diameter × 30 cm height) filled with water at 23 ± 1°C which was made white opaque with powdered milk (Cat # 902887, MP Biomedicals, Solon, OH). The hidden platform (5 cm diameter × 18 cm height) was submerged 1.5 cm below the water surface. The maze was located in a 2.5 × 1.4 m room and the geometric signs of triangle, square, and circle were used on the wall as spatial cues. The maze was divided into four quadrants (north, south, east, and west). The platform was placed in the middle of the east quadrant and remained in the same position during acquisition. During acquisition, each animal had 4 trials daily at 1 min intervals for 7 days. Each animal was released from 1 of the quadrants facing the wall of the pool. The order of the start location was rotated every trial as south-west-north-south and each trial lasted until the animal found the platform within 60 s and remained on the platform for 30 s. If the animal failed to find the platform within 60 s, it was placed on the platform for 30 s by the researcher. At the end of each trial, mice were removed, towel dried, and placed in a cage under a heating lamp until the next trial.
A visible platform (2.5 cm above the water surface) test was performed at the beginning of training (day 1) and end of the last session (day 7) to identify whether there were any visual or motor deficits with mice that impaired their performance. Similar results were observed in both tests and data from the last test are presented.
BrdU administration and cell proliferation assay
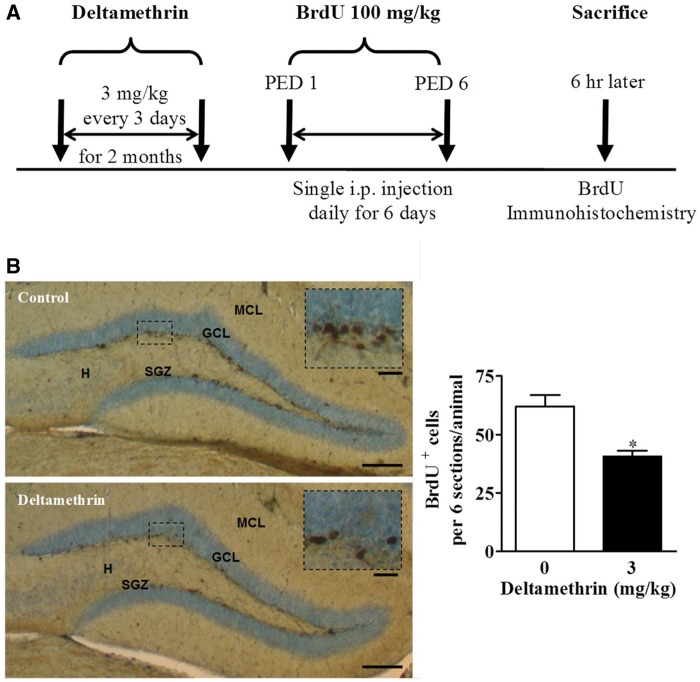
To assess cell proliferation, mice received daily 100 mg/kg IP injections of 5-bromo-2-deoxyuridine (BrdU) for 6 consecutive days starting 24 h after the last dose of deltamethrin (Figure 6A). BrdU is an analog of thymidine, and incorporates into the newly synthesized DNA in S-phase of the mitosis and labels proliferating cells (Falluel-Morel et al., 2007; Mishra et al., 2012; Winocur et al., 2006). Mice were killed 6 h after the last BrdU injection. Brains were then removed, fixed in 4% paraformaldehyde as above, and 30 μm coronal sections were cut through the entire hippocampus. Free floating sections were incubated in 2 N HCl for 30 min at room temperature to denature DNA, followed by incubation with mouse monoclonal anti-BrdU (1:200; cat # 5292, Cell Signaling) according to the procedure described above.
FIG. 6.
Repeated deltamethrin exposure reduced the number of BrdU+ cells in the dentate gyrus (DG) in hippocampus of adult mice. A, Experimental paradigm used to examine proliferation; PED, post-exposure day, B, Immunostaining of BrdU (dark brown) in the DG region of the hippocampus. BrdU-positive cells in DG were significantly decreased following long-term low-level deltamethrin exposure to adult mice. The values represent mean cell number ± SEM per section from 6 sections per animal and 3 animals per group. Asterisk indicates significantly different from control (t = 3.91; df = 4; P = .02). Hhilus; GCL, granule cell layer; SGZ, sub granular zone; MCL, molecular cell layer. Scale bar = 1600 μm (B) and 200 μm in inset.
BrdU+ cell quantification
Following immunohistochemical labeling, BrdU+ cells in the bilateral dentate gyrus subgranular zone (SGZ) of each brain were counted from a total of 6 sections per animal and 3 animals per group. Then the average number of BrdU+ cells from the 6 sections for each animal was the averaged to obtain the group mean, and compared by t-test.
Statistical analysis
Statistical analyses were performed using Prism 5.01 software (GraphPad, San Diego, CA). Data are presented as mean ± SEM. Data for the neurochemical and histological assays, data were analyzed by an unpaired t-test with Welch’s correction. Body weights were analyzed by 2-way ANOVA. For the Morris maze, daily blocks from the average of 4 trials within each day were used for analysis. A 2-way repeated measures ANOVA was then used to determine main effects (treatment) with day as the repeated measure and latency as the dependent variable.
RESULTS
General Appearance and Body Weight
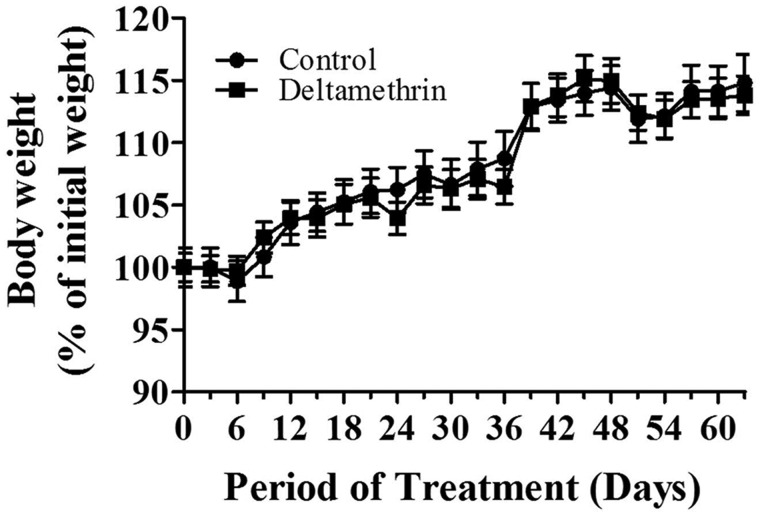
Mice treated with deltamethrin for 60 days showed no signs of toxicity such as tremor, salivation, and ataxia. Likewise, deltamethrin did not cause deficits in weight gain, as animals in both groups gained weight over time at a similar rate (Figure 1).
FIG. 1.
Repeated deltamethrin exposure does not cause changes in weight gain during treatment. Body weight was recorded prior to dosing. The initial average body weight for control animals was 30.08 ± 0.47 (g) and for deltamethrin animals was 28.68 ± 0.17 (g). The values represent mean ± SEM from 12 to14 animals per group.
Activation of the ER Stress Pathway in Hippocampus Following Repeated Exposure to Deltamethrin
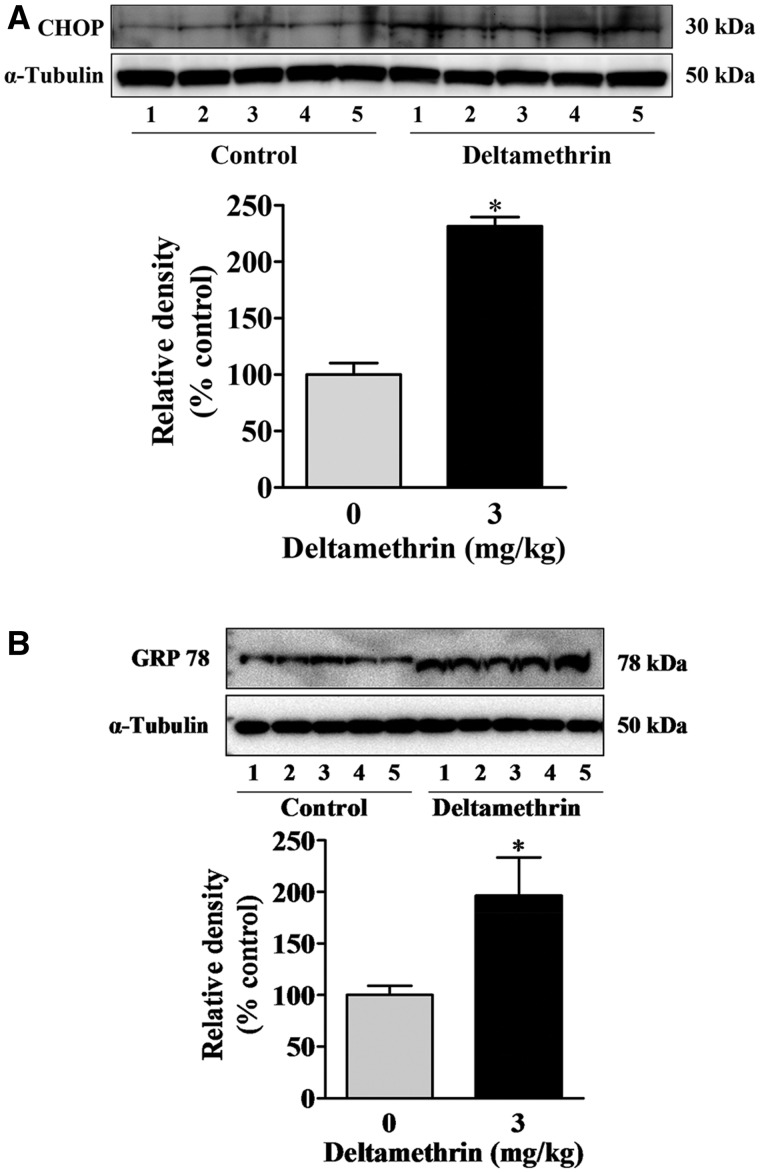
To determine whether deltamethrin induced ER stress in vivo, ER stress-related proteins in the hippocampus were quantified by Western blot analysis 3 days after final exposure. Repeated exposure to deltamethrin significantly increased the ER stress-related proteins CHOP (131%) and GRP78 (96%) in hippocampus (Figure 2A and B).
FIG. 2.
Repeated deltamethrin exposure causes ER stress in the hippocampus of adult mice. Induction of CHOP (A) and GRP78 (B) proteins at 3 days following last deltamethrin exposure was determined by Western blot analysis. α-Tubulin was used as loading control. Relative densities are presented in bar graphs and representative blots are provided above the graphs. The values represent mean ± SEM from 5 animals per group. Asterisk indicates significantly different from control (t = 10.01; df = 8; P < .001 for CHOP and t = 2.53; df = 8; P = .02 for GRP78).
Repeated Exposure to Deltamethrin Causes ER-Stress Mediated Apoptosis in the Hippocampus
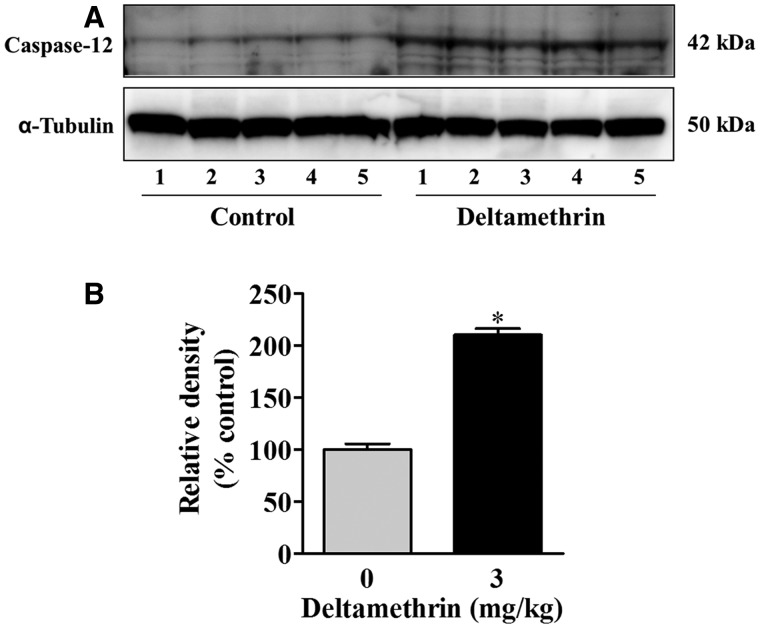
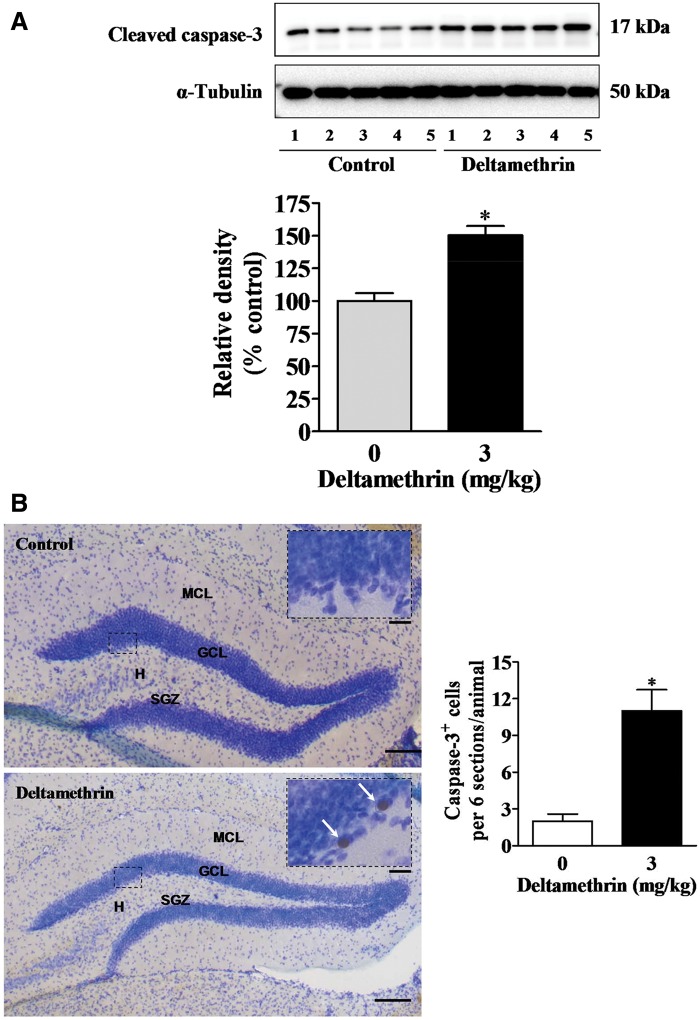
We measured protein levels of hippocampal caspases, as ER stress-mediated apoptotic death signaling can occur through the sequential activation of caspase-12 and subsequently caspase-3. A 2-fold increase in caspase-12 was found in the hippocampus of deltamethrin-treated animals (Figure 3). Deltamethrin exposure also significantly increased the protein levels of activated caspase-3 by 50% in the hippocampus as compared with control (Figure 4A). To further define the localization of cells undergoing apoptosis, we performed immunohistochemistry to examine the dentate gyrus SGZ. There was a 4-fold increase in the number of cleaved caspase-3 immunoreactive cells observed in the dentate gyrus following deltamethrin exposure, primarily localized to the SGZ (Figure 4B).
FIG. 3.
Repeated deltamethrin exposure activates caspase-12 in the hippocampus of adult mice. A, Deltamethrin increased protein levels of caspase-12 in the hippocampus. B, Relative densities from representative blots are presented in bar graphs. α-Tubulin was used as loading control for Western blot analysis. The values represent mean ± SEM from 5 animals per group. Asterisk indicates significantly different from control (t = 12.98; df = 8; P < .0001).
FIG. 4.
Repeated deltamethrin exposure activates caspase-3 in the hippocampus of adult mice. A, Western blot analysis was used to assess cleaved caspase-3 protein levels in the whole hippocampus 3 days after the last deltamethrin exposure. α-Tubulin was used as loading control. Relative densities are presented in bar graphs and a representative blot is provided above the graph. The values represent mean ± SEM from 5 animals per group. Asterisk indicates significantly different from control t = 5.35; df = 8; P = .003). B, Caspase-3 immunoreactive cells were visualized by immunostaining in the dentate gyrus (DG) of hippocampus. Arrows point to caspase-3+ labeled cells. H, Hilus of the dentate gyrus; GCL, granule cell layer; SGZ, sub granular zone; MCL, molecular cell layer. Scale bar = 1600 μm (B) and 200 μm in inset. Data are from 3 animals per group. Asterisk indicates significantly different from control (t = 4.93; df = 4; P = .008).
Impairment of Learning in Adult Mice Following Repeated Treatment with Deltamethrin
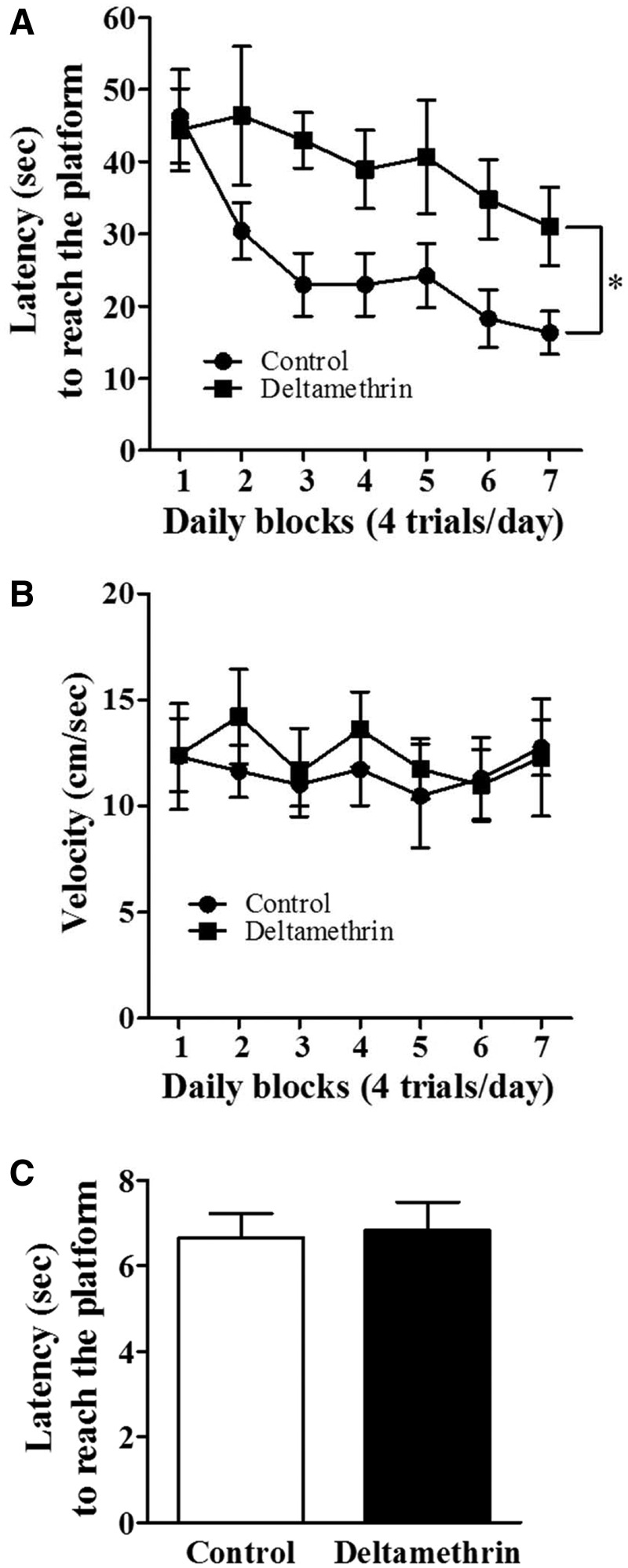
To determine whether deltamethrin exposure and ER stress were associated with cognitive dysfunction, hippocampal-dependent learning was evaluated in mice using the Morris water maze. In this task, animals were trained to find the hidden platform in a pool of opaque water. Mice learned the location of the platform by using visual cues outside the maze for 7 days. Control animals showed daily improvements in their abilities to find the hidden platform during the acquisition phase, whereas deltamethrin-treated animals showed an impaired acquisition process (Figure5A). The difference between groups was not the result of motor or visual impairment, because neither swim speed (Figure 5B) nor visual acuity (Figure 5C) was affected by deltamethrin treatment.
FIG. 5.
Repeated deltamethrin exposure causes deficits in hippocampal-dependent learning in adult mice. A, Latency to find hidden platform; B, Swim speed; and C, Visual platform test. Data represent mean ± SEM of 6 individual animals. Asterisk indicates significant treatment effect (F = 23.70; df = 1,6; P = .0001).
Repeated Deltamethrin Exposure Decreases Cell Proliferation in the Dentate Gyrus
To examine the effects of deltamethrin on cell proliferation, mice were given a single BrdU injection daily for 6 days and sacrificed 6 h after the last BrdU injection (Figure 6A). Deltamethrin significantly reduced the number of BrdU-positive cells (30%) in the dentate gyrus (Figure 6B).
DISCUSSION
ER stress is a significant contributor to neurodegeneration and cognitive dysfunction in a variety of diseases, including Alzheimer’s disease, Parkinson’s disease, and Amyotrophic lateral sclerosis (Hoozemans et al., 2009; Lindholm et al., 2006; Prell et al., 2014; Salminen et al., 2009; Zhang et al., 2013). Previously, we reported that the pyrethroid pesticide deltamethrin causes ER stress and apoptosis in vitro (Hossain and Richardson, 2011). In this study, we demonstrate that repeated exposure to low levels of deltamethrin causes hippocampal ER stress and deficits in learning in adult mice. These deficits were accompanied by a marked reduction of BrdU+ cells and increased cell death in the hippocampal dentate gyrus, suggesting that repeated deltamethrin exposure may inhibit hippocampal neurogenesis by reducing neuronal progenitor cell proliferation.
To evaluate ER stress following long-term deltamethrin exposure to mice, we measured the ER-specific chaperone GRP78 and the transcription factor CHOP in the hippocampus. GRP78 and CHOP are normally expressed at very low levels under physiological conditions but they are increased under conditions of ER stress (Oyadomari and Mori, 2004). CHOP decreases expression of the anti-apoptotic molecule Bcl-2 (Xu et al., 2005) and increases the expression of various pro-apoptotic molecules including DR5, TRB3, and GADD34 in ER-stressed cells (Zhang and Kaufman, 2008). The balance between pro-apoptotic and anti-apoptotic molecules plays a role in regulating the transition from a protective to an apoptotic response. Our results demonstrate that repeated exposure to low levels of deltamethrin caused ER stress as both GRP78 and CHOP were significantly increased in the hippocampus.
Activation of caspase-3 is a critical event in the execution phase of apoptosis involving pesticide exposure and occurs through mitochondrial dysfunction or ER stress pathways, depending on the origin of the death stimulus (Choi et al., 2010; Hossain and Richardson, 2011; Kitazawa et al., 2003; Ramachandiran et al., 2007; Tait and Green, 2010). In our previous study, we found that deltamethrin caused apoptosis in neuroblastoma cells through the ER stress pathway (Hossain and Richardson, 2011). Caspase-12 is an up-stream cysteine protease normally found in an inactive form in the ER and activated during ER stress (Nakagawa et al., 2000). In the ER stress pathway, apoptotic cell death is different from the intrinsic pathway because caspase-12 can directly activate the downstream caspase-9 without release of cytosolic cytochrome c from mitochondria (Hossain and Richardson, 2011, Morishima et al., 2002). In this study, an increase in activated caspase-3 was accompanied by up-regulation of caspase-12 in the hippocampus of deltamethrin-treated mice, supporting the hypothesis that deltamethrin causes apoptotic neuronal cell death in hippocampus through activation of the ER stress pathway, similar to that observed in vitro (Hossain and Richardson, 2011).
Cumulative exposure to pesticides is thought to pose considerable health risks, including behavioral dysfunction (Rohlman et al., 2007), yet few data are available regarding the potential effects of pyrethroid pesticides. There are two previous studies involving deltamethrin exposure of adult rats that reported cognitive effects in the Y-maze (Husain et al., 1996) and Morris Water Maze (Chen et al., 2012), but both used much higher doses, 7 and 12.5 mg/kg/day. Here, we found that mice repeatedly exposed to deltamethrin exhibited deficits in hippocampal-dependent learning as assessed by the Morris Water Maze. Our results further demonstrated that animals were not impaired in the visible platform task, indicating that the deficit in learning was not the result of impaired vision or motor function following deltamethrin treatment. Taken together with our finding of increased ER stress, this finding is consistent with recent literature data reporting that sustained ER stress is associated with learning deficits (Hotamisligil, 2010; Salminen et al., 2009). Additionally, Zhang et al. (2013) reported that ER stress-mediated apoptosis in hippocampus produces a deficit in learning in diabetic rats. Thus, our data suggest that persistent hippocampal ER stress following long-term low-levels of pyrethroids exposure may contribute to neuronal dysfunction leading to learning deficits in mice.
It is becoming increasingly recognized that exposure to environmental toxicants, including pesticides, can lead to disruption of neurogenesis and cognitive deficits, particularly following developmental exposures (Falluel-Morel et al., 2007; Mishra et al., 2012; Ojo et al., 2014; Sokolowski et al., 2013). Further, disruption of hippocampal neurogenesis is an important mediator of cognitive function (Winocur et al., 2006; Zhao et al., 2008). The adult-born cells in DG migrate to the granule cell layer and differentiate into mature neurons whose projections along the mossy fibers extend to the CA3 region in the hippocampus (Cameron et al., 1993; Stanfield and Trice, 1988; Thuret et al., 2009). Other studies demonstrate that adult-born neurons in the DG are functionally integrated into the existing neuronal circuitry and contribute to hippocampal-dependent learning and memory (Lemaire et al., 2000; Lu and Chang, 2004; Snyder et al., 2005; Thuret et al., 2009; Zhao et al., 2008). Here, we found that deltamethrin decreased the number of BrdU+ cells, those engaged in mitotic S-phase, in the DG of hippocampus, suggesting that deltamethrin may reduce hippocampal neurogenesis by damaging the neuronal progenitor cells (NPCs) which are thought to be involved in learning processes in the adult hippocampus (Falluel-Morel et al., 2007; Shors et al., 2002; Snyder et al., 2005). However, additional studies will be required to determine the relative contributions of reduced cell proliferation and/or enhanced cell death in the hippocampal abnormalities that follow deltamethrin exposure.
In conclusion, this study provides evidence that long-term exposure to relatively low levels of deltamethrin causes ER stress and apoptotic cell death in the hippocampus of adult mice. This was accompanied by a significant reduction of cell proliferation and increased apoptosis in the dentate gyrus of deltamethrin-treated mice. Therefore, it is conceivable that the impairment of hippocampal neurogenesis by deltamethrin may contribute to the observed deficits in learning in mice. In addition, the hippocampus seems may be particularly vulnerable to pyrethroids, as Na+ current and Na+ channel density is higher in the hippocampus than other brain regions (Xia et al., 2003). Taken together, these findings raise a concern that long-term human exposure to pyrethroid pesticides could contribute to deficits in learning. Further studies are required to confirm impairment of hippocampal neurogenesis and identify the precise mechanism(s) by which long-term adult exposure to low levels of pyrethroid pesticides contribute to deficits in learning.
FUNDING
This work was supported in part by grants from National Institutes of Health [grant nos. R01ES015991, R01ES021800, P30ES005022, R21NS072097, and U01NS079249].
REFERENCES
- Aprea C., Stridori A., Sciarra G. (1997). Analytical method for the determination of urinary 3-phenoxybenzoic acid in subjects occupationally exposed to pyrethroid insecticides. J. Chromatogr. B 695, 227–236. [DOI] [PubMed] [Google Scholar]
- Berkowitz G. S., Obel J., Deych E., Lapinski R., Godbold J., Liu Z., Landrigan P. J., Wolff M. S. (2003). Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ. Health Persp. 111, 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert G. M., Mehler L. N., Rosales R., Baum L., Thomsen C., Male D., Shafey O., Das R., Lackovic M., Arvizu E. (2003). Acute pesticide-related illnesses among working youths, 1988–1999. Am J. Public Health 93, 605–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron H. A., Woolley C. S., McEwen B. S., Gould E. (1993). Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience 56, 337–344. [DOI] [PubMed] [Google Scholar]
- Chen N. N., Luo D. J., Yao X. Q., Yu C., Wang Y., Wang Q., Wang J. Z., Liu G. P. (2012). Pesticides induce spatial memory deficits with synaptic impairments and an imbalanced tau phosphorylation in rats. J. Alzheimers Dis. 30, 585–594. [DOI] [PubMed] [Google Scholar]
- Choi W. S., Abel G., Klintworth H., Flavell R. A., Xia Z. (2010). JNK3 mediates paraquat- and rotenone-induced dopaminergic neuron death. J. Neuropathol. Exp. Neurol. 69, 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow J. A., Borazjani A., Potter P. M., Ross M. K. (2007). Hydrolysis of pyrethroids by human and rat tissues: examination of intestinal, liver, and serum carboxylesterases. Toxicol. Appl. Pharmacol. 221, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwan M. A., Richardson J. R., Guillot T. S., Caudle W. M., Miller G. W. (2006). Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol. Appl. Pharmacol. 211, 188–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Environmental Protection Agency (2010). Deltamethrin. Human health assessment scoping document in support of registration review. Available at: www.epa.gov/espp/litstatus/effects/redleg-frog/2013/deltamethrin/appendix-j.pdf. [Google Scholar]
- Falluel-Morel A., Sokolowski K., Sisti H. M., Zhou X., Shors T. J., Dicicco-Bloom E. (2007). Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J. Neurochem. 103, 1968–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin S. J., Crow J. A., Scollon E. J., Hughes M. F., DeVito M. J., Ross M. K. (2007). Identification of rat and human cytochrome p450 isoforms and a rat serum esterase that metabolize the pyrethroid insecticides deltamethrin and esfenvalerate. Drug Metab. Dispos. 35, 1664–1671. [DOI] [PubMed] [Google Scholar]
- Godin S. J., DeVito M. J., Hughes M. F., Ross D. G., Scollon E. J., Starr J. M., Setzer R. W., Conolly R. B., Tornero-Velez R. (2010). Physiologically based pharmacokinetic modeling of deltamethrin: development of a rat and human diffusion-limited model. Toxicol. Sci. 115, 330–343. [DOI] [PubMed] [Google Scholar]
- Hoozemans J. J., van Haastert E. S., Nijholt D. A., Rozemuller A. J., Eikelenboom P., Scheper W. (2009). The unfolded protein response is activated in pretangle neurons in Alzheimer's disease hippocampus. Am. J. Pathol. 174, 1241–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain M. M., Richardson J. R. (2011). Mechanism of pyrethroid pesticide-induced apoptosis: Role of calpain and the ER stress pathway. Toxicol. Sci. 122, 512–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S. (2010). Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain R., Husain R., Adhami V. M., Seth P. K. (1996). Behavioral, neurochemical, and neuromorphological effects of deltamethrin in adult rats. J. Toxicol. Environ. Health 48, 515–526. [DOI] [PubMed] [Google Scholar]
- Kimata A., Kondo T., Ueyama J., Yamamoto K., Yoshitake J., Takagi K., Suzuki K., Inoue T., Ito Y., Hamajima N., et al. (2009). Comparison of urinary concentrations of 3-phenoxybenzoic acid among general residents in rural and suburban areas and employees of pest control firms. Int. Arch. Occ. Environ. Health 82, 1173–1178. [DOI] [PubMed] [Google Scholar]
- Kitazawa M., Anantharam V., Kanthasamy A. G. (2003). Dieldrin induces apoptosis by promoting caspase-3-dependent proteolytic cleavage of protein kinase Cdelta in dopaminergic cells: Relevance to oxidative stress and dopaminergic degeneration. Neuroscience 119, 945–964. [DOI] [PubMed] [Google Scholar]
- Lemaire V., Koehl M., Le Moal M., Abrous D. N. (2000). Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl Acad. Sci. USA 97, 11032–11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm D., Wootz H., Korhonen L. (2006). ER stress and neurodegenerative diseases. Cell Death Differ. 13, 385–392. [DOI] [PubMed] [Google Scholar]
- Lu B., Chang J. H. (2004). Regulation of neurogenesis by neurotrophins: Implications in hippocampus-dependent memory. Neuron Glia Biol. 1, 377–384. [DOI] [PubMed] [Google Scholar]
- Mishra D., Tiwari S. K., Agarwal S., Sharma V. P., Chaturvedi R. K. (2012). Prenatal carbofuran exposure inhibits hippocampal neurogenesis and causes learning and memory deficits in offspring. Toxicol. Sci. 127, 84–100. [DOI] [PubMed] [Google Scholar]
- Morgan M. K. (2012). Children's exposures to pyrethroid insecticides at home: A review of data collected in published exposure measurement studies conducted in the United States. Int. J. Environ. Res. Pub. Health 9, 2964–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima N., Nakanishi K., Takenouchi H., Shibata T., Yasuhiko Y. (2002). An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J. Biol. Chem. 277, 34287–34294. [DOI] [PubMed] [Google Scholar]
- Morris R. (1984). Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 11, 47–60. [DOI] [PubMed] [Google Scholar]
- Muller-Mohnssen H. (1999). Chronic sequelae and irreversible injuries following acute pyrethroid intoxication. Toxicol. Lett. 107, 161–176. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Zhu H., Morishima N., Li E., Xu J., Yankner B. A., Yuan J. (2000). Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-beta. Nature 403, 98–103. [DOI] [PubMed] [Google Scholar]
- Ojo J. O., Abdullah L., Evans J., Reed J. M., Montague H., Mullan M. J., et al. (2014). Exposure to an organophosphate pesticide, individually or in combination with other Gulf War agents, impairs synaptic integrity and neuronal differentiation, and is accompanied by subtle microvascular injury in a mouse model of Gulf War agent exposure. Neuropathology 34, 109–127. [DOI] [PubMed] [Google Scholar]
- Oyadomari S., Mori M. (2004). Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 11, 381–389. [DOI] [PubMed] [Google Scholar]
- Prell T., Lautenschlager J., Weidemann L., Ruhmer J., Witte O. W., Grosskreutz J. (2014). Endoplasmic reticulum stress is accompanied by activation of NF-kappaB in amyotrophic lateral sclerosis. J. Neuroimmunol. 270, 29–36. [DOI] [PubMed] [Google Scholar]
- Ramachandiran S., Hansen J. M., Jones D. P., Richardson J. R., Miller G. W. (2007). Divergent mechanisms of paraquat, MPP+, and rotenone toxicity: Oxidation of thioredoxin and caspase-3 activation. Toxicol. Sci. 95, 163–171. [DOI] [PubMed] [Google Scholar]
- Rohlman D. S., Lasarev M., Anger W. K., Scherer J., Stupfel J., McCauley L. (2007). Neurobehavioral performance of adult and adolescent agricultural workers. Neurotoxicology 28, 374–380. [DOI] [PubMed] [Google Scholar]
- Salminen A., Kauppinen A., Suuronen T., Kaarniranta K., Ojala J. (2009). ER stress in Alzheimer's disease: A novel neuronal trigger for inflammation and Alzheimer's pathology. J. Neuroinflammation 6, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sama D. M., Norris C. M. (2013). Calcium dysregulation and neuroinflammation: Discrete and integrated mechanisms for age-related synaptic dysfunction. Ageing Res. Rev. 12, 982–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors T. J., Townsend D. A., Zhao M., Kozorovitskiy Y., Gould E. (2002). Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus 12, 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. (1985). Measurement of protein using bicinchoninic acid. Anal Biochem. 150, 76–85. [DOI] [PubMed] [Google Scholar]
- Snyder J. S., Hong N. S., McDonald R. J., Wojtowicz J. M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852. [DOI] [PubMed] [Google Scholar]
- Sokolowski K., Obiorah M., Robinson K., McCandlish E., Buckley B., DiCicco-Bloom E. (2013). Neural stem cell apoptosis after low-methylmercury exposures in postnatal hippocampus produce persistent cell loss and adolescent memory deficits. Dev. Neurobiol. 73, 936–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanfield B. B., Trice J. E. (1988). Evidence that granule cells generated in the dentate gyrus of adult rats extend axonal projections. Exp. Brain Res. 72, 399–406. [DOI] [PubMed] [Google Scholar]
- Tait S. W., Green D. R. (2010). Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 11, 621–632. [DOI] [PubMed] [Google Scholar]
- Thuret S., Toni N., Aigner S., Yeo G. W., Gage F. H. (2009). Hippocampus-dependent learning is associated with adult neurogenesis in MRL/MpJ mice. Hippocampus 19, 658–669. [DOI] [PubMed] [Google Scholar]
- Trunnelle K. J., Bennett D. H., Ahn K. C., Schenker M. B., Tancredi D. J., Gee S. J., Stoecklin-Marois M. T., Hammock B. D. (2014). Concentrations of the urinary pyrethroid metabolite 3-phenoxybenzoic acid in farm worker families in the MICASA study. Environ. Res. 131, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama J., Kimata A., Kamijima M., Hamajima N., Ito Y., Suzuki K., Inoue T., Yamamoto K., Takagi K., Saito I., et al. (2009).Urinary excretion of 3-phenoxybenzoic acid in middle-aged and elderly general population of Japan. Environ. Res. 109, 175–180. [DOI] [PubMed] [Google Scholar]
- Winocur G., Wojtowicz J. M., Sekeres M., Snyder J. S., Wang S. (2006). Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus 16, 296–304. [DOI] [PubMed] [Google Scholar]
- Wu A., Li L., Liu Y. (2003). Deltamethrin induces apoptotic cell death in cultured cerebral cortical neurons. Toxicol. Appl. Pharmacol. 187, 50–57. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y. (2000). Apoptotic cell death in rat brain following deltamethrin treatment. Neurosci. Lett. 279, 85–88. [DOI] [PubMed] [Google Scholar]
- Xia Y., Zhao P., Xue J., Gu X. Q., Sun X., Yao H., et al. (2003). Na+ channel expression and neuronal function in the Na+/H+ exchanger 1 null mutant mouse. J. Neurophysiol. 89, 229–236. [DOI] [PubMed] [Google Scholar]
- Xu C., Bailly-Maitre B., Reed J. C. (2005). Endoplasmic reticulum stress: Cell life and death decisions. J. Clin. Investig. 115, 2656–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Kaufman R. J. (2008). From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu L., He D., Ling S. (2013). Endoplasmic reticulum stress-mediated hippocampal neuron apoptosis involved in diabetic cognitive impairment. BioMed. Res. Int. 2013, 924327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Deng W., Gage F. H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660. [DOI] [PubMed] [Google Scholar]