Abstract
Background:
The aim was to evaluate effects of 5-week whole body vibration (WBV) training with different amplitudes and progressive frequencies on fibrinolytic/coagulative factors.
Materials and Methods:
25 subjects were divided randomly in high or low-amplitude vibration, and control groups. Training consisted of 5-week WBV with amplitudes 4 or 2 mm. Plasma samples were analyzed before and after training. Statistical analysis was done using one-way analysis of variance and Wilcoxon signed ranked test. P <0.05 was considered significant.
Results:
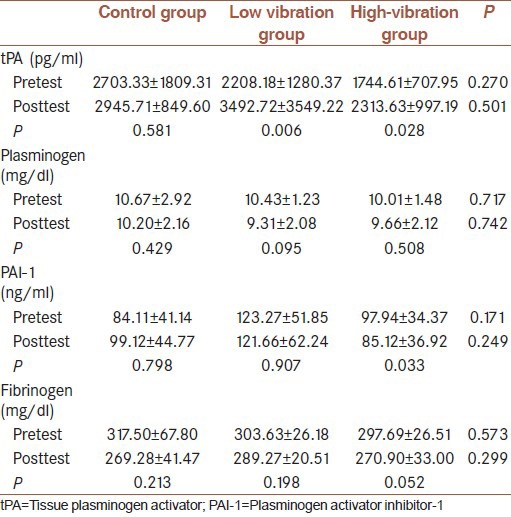
High-amplitude vibration caused an increase in tissue plasminogen activator (tPA) (P = 0.028) (pretest: 1744.61 ± 707.95; posttest: 2313.63 ± 997.19 pg/ml), and decrease in plasminogen activator inhibitor-1 (PAI-1) (P = 0.033) (pretest: 97.94 ± 34.37; posttest: 85.12 ± 36.92 ng/ml). Fibrinogen and plasminogen were not changed significantly. Low-amplitude vibration caused an increase in tPA (P = 0.006) (pretest: 2208.18 ± 1280.37; posttest: 3492.72 ± 3549.22 pg/ml). PAI-1, fibrinogen and plasminogen were not changed significantly. There were no significant differences between groups.
Conclusion:
Amplitude of vibrations in WBV training may affect fibrinolytic factors.
Keywords: Blood coagulation, fibrinolysis, vibration
INTRODUCTION
Sedentary life style may cause cardiovascular disorders through multiple pathophysiological mechanisms involving the coagulation system.[1] In most cardiac events, thrombosis has a fundamental role in plaque propagation and commencing acute coronary syndromes.[2] Coagulation and fibrinolysis are opposite physiological mechanisms in homeostasis.[3,4] The coagulation system evolves into fibrin formation, however, fibrinolytic mechanism leads to dissolving fibrin clots.[3,5,6]
The prevalence of coronary artery diseases is increasing in the young population. Lack of classical risk factors (hyperlipidemia, hypertension, and diabetes mellitus) in some young people affected by myocardial infarction supports the theory that imbalance in the homeostasis system may be a possible etiology for thrombus formation and myocardial infarction.[7]
Balance between homeostatic potentials and thrombogenic factors through simple changes in lifestyle may be considered as primary or secondary prevention methods. One of the changes stressed by researchers is exercise and physical activity. El-Sayed et al. showed an increase in coagulation activity in response to a 12-week moderate intensity training program.[8] Koenig and Ernst in a study on relationship between plasma fibrinogen level, physical activity and aerobic performance found a significant decrease in fibrinogen level after a 12-month period.[9] Hilberg et al. declared that in healthy individuals acute high-intensity exercise increases coagulation system parameters just in normal range; on the contrary, the facilitation in fibrinolysis system is more profound and related to duration of exercise.[10]
Hilberg et al. found that endurance exercise with an intensity below 90% individual anaerobic threshold and a duration below 2 h generates a more favorable condition for fibrinolysis than for blood coagulation in healthy young subjects.[11]
Physical activity leads to decreased cardiovascular risk factors. In addition to improvement in cardiovascular performance and increased physical fitness, it causes a balance in homeostatic system. In the literature, due to various training protocols (intensity, duration, and frequency) many controversies can be found. In many studies high-intensity exercise increased coagulation system activity; on the contrary, moderate intensity exercise increased the fibrinolysis system activity.[8,12,13,14,15] Resistance exercises also proved effective on the mentioned systems.[16,17]
Whole body vibration (WBV) is a novel modality in which subjects stand on a platform, in a static position or performing dynamic exercises, that moves at a predetermined frequency and amplitude, transmitting the vibration from the platform up through the feet and legs. Studies have utilized training time periods ranging from 10 days to 6 months, frequencies between 12 and 60 Hz, and amplitudes ranging from 1.7 to 10 mm.[18,19] As the movement of the platform is sinusoidal, the acceleration transmitted to the body is calculated as a = A (2πf)2. Where “A” is the amplitude of the oscillations and “f” is the frequency.[20] Small changes in amplitude and frequency determine relatively large changes in acceleration and magnitude of vibration being transmitted to the body. The aim of the present study was to assess effects of 5-week WBV training with different amplitudes on fibrinolytic and coagulative activities.
MATERIALS AND METHODS
Subject selection
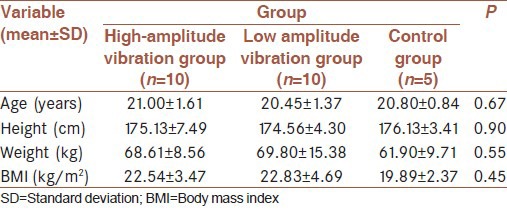
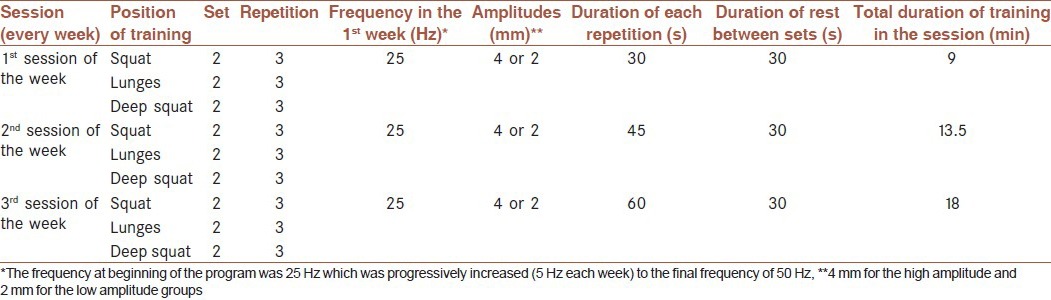
In this interventional study, a total of 35 men (aged 19-35 years) were candidate for study (as inclusion criteria) who had not done any exercises regularly for at least 3 months before the start of the study served as subjects. Men with cardiovascular or coagulative problems were excluded (five person). All subjects were informed of the procedures, risks and benefits in advance. They were divided randomly into three groups. Five subjects were excluded because they were not participate in posttest in control group measurements. Distribution of remaining subjects completing exercise sessions consisted of high-amplitude vibration group (n = 10), low-amplitude vibration group (n = 10), and control group (n = 5). The vibration training consisted of 5-week whole-body vibration 3 times a week with amplitudes of 4 and 2 mm and progressive frequencies from 25 Hz with increments of 5 Hz weekly. Table 1 shows descriptive features of the sample.
Table 1.
The descriptive features of the sample
Vibration training
During the 5-week experimental period all subjects continued their conventional living schedule; but those in the WBV groups additionally performed 3 times weekly WBV training on a vibration platform (Fit Vib, Germany).
Vibration groups were divided into low-amplitude (2 mm) and high-amplitude (4 mm) groups with progressively increasing frequencies. The training programs are summarized in Table 2.
Table 2.
The training schedule
Blood samples were taken from all subjects after 12-14 h of overnight fasting from antecubital vein in a similar condition. Concentrations of fibrinogen (Clauss, Mahsa Yaran, Iran), plasminogen (ELISA, Cusabio, China), tissue plasminogen activator (tPA) (ELISA, Cusabio, China), and plasminogen activator inhibitor-1 (PAI-1) (ELISA, Cusabio, China) before and after 5-week of training were measured in plasma samples.
Statistical analysis
In order to check the normality of data Kolomogrov–Smirnov test and for homogeneity of variances Leven test were used. If the normality of data or homogeneity of variances assumption not held we used nonparametric Kruskal–Wallis test. Otherwise we used analysis of variance for comparing groups. In order to compare pretest with posttest we used Wilcoxon signed ranked test. Data are expressed as mean ± standard deviation. The level of significance was set at P < 0.05.
Ethical issues
The study protocol was evaluated and accepted by Ethical Committee of the Sports Medicine Federation of Iran and it complies with Helsinki declaration. All the participants received adequate information about the study protocol and possible good and ill effects of training. They entered the study deliberately and were free to quit the protocol upon their request.
RESULTS
The 5-week high-amplitude vibration training caused a significant improvement in tPA (P = 0.028), and PAI-1 (P = 0.033), fibrinogen showed decrease albeit not significantly (P = 0.052). Plasminogen showed decrease not significantly (P = 0.508). Low-amplitude vibration training caused a significant improvement in tPA (P = 0.006) and and PAI-1 showed decrease not significantly (P = 0.907). Fibrinogen showed decrease albeit not significantly (P = 0.19). Plasminogen showed decrease not significantly (P = 0.095). However, between groups there was no significant effect on tPA (P = 0.50), PAI-1 (P = 0.249), plasminogen (P = 0.742), and fibrinogen (P = 0.299). The control group does not show any significant change in the variables studied.
The data of, tPA, fibrinogen, PAI-1 and plasminogen before and after low and high-vibration training are showed in Table 3.
Table 3.
Data of tPA, plasminogen, PAI-1 and fibrinogen before and after low and high-vibration training and control groups
DISCUSSION
Coagulation and fibrinolysis are opposite dynamic processes important in homeostasis. Plasminogen, synthesized in the liver, is the inactive form of plasmin. Once activated, plasmin digests fibrin breaking it into fibrin dimer proteins that can then be cleared by the liver. tPA is the most abundant activator of fibrinolysis. tPA is synthesized, stored, and released from endothelial cells in the vasculature, sympathetic neurons and skeletal muscle.[21] PAI-1 is a protein that is the primary inhibitor of tPA and is synthesized by endothelial cells and platelets which regulate its release during fibrinolysis.[22] Fibrinogen is one of the essential factors in the coagulation process. Fibrinogen is a high-molecular weight protein and is formed in the liver.[23]
Immediately following an acute bout of both aerobic and resistance exercise, fibrinolytic activity is shown to increase as evidenced by an increase in the plasma concentration of active tPA and decrease in plasma PAI-1 resulting in the potential breakdown of clots.[24] El-Sayed et al. showed an increase in coagulation activity in response to a 12-week moderate intensity training program.[8]
The use of WBV as an exercise method has rapidly increased over the last decade. Variables of vibration training like other training protocols consist of frequency (Hz), amplitude (mm), and duration (s). Rittweger et al. reported that oxygen uptake during standing and squatting exercise with WBV was greater than that without WBV. An increase in the level of muscle activity caused by WBV affects the cardiovascular system. WBV has been shown to cause muscle activation.[20] Mechanical vibration applied to the muscle belly, tendon or whole body had been shown to elicit a tonic vibration reflex.[25,26,27,28]
A significantly higher level of electromyographic activity of the vastus lateral is muscle was found in a half squat position during WBV than that in a nonvibrating condition.[29] Skeletal muscle is known to be a release site of tPA, suggesting that skeletal muscle activation in WBV may stimulate the muscle to release tPA. The mechanism underlying the fibrinolytic responses reported in the present study may be related to vascular shear stress. Kerschan-Schindl et al. reported that muscular blood circulation in the calf and thigh significantly increased after one bout of WBV exercise. A few minutes lasting stance on vibrating platform leads to an increase in the relative moving blood volume of quadriceps and gastrocnemius muscles. Mean blood flow in the popliteal artery was also increased and its resistive index decreased.[30] These results suggest that WBV causes an increase in blood volume after squatting exercise. In a study on 20 healthy young men Boyle and Nagelkirk concluded that WBV combined with squatting exercise increases fibrinolytic activity more than exercise alone.[31] During WBV exercise there is an enhancement of skin blood flow, as measured by laser Doppler flowmetry.[32,33,34] This increase in blood flow may result in an increase in shear stress on blood vessels. Increased blood flow, which may result in increased stress on endothelial walls of blood vessels, has also been shown with WBV; leading to the speculation that WBV may also result in an increased fibrinolytic response.[30] Thus, WBV may affect fibrinolytic activity and enhance the cardio-protective benefits of a therapeutic exercise regimen. Increased vascular shear stress has been shown to increase fibrinolytic activity in cultured endothelial cells.[10]
The mechanism underlying the fibrinolytic responses reported in the present study may be related to vascular shear stress. As the movement of the platform is sinusoidal, the acceleration transmitted to the body is calculated as a = A (2πf)2. Where “A” is the amplitude of the oscillations and “f” is the frequency 4. Small changes in amplitude and frequency determine relatively large changes in acceleration and magnitude of vibration being transmitted to the body. The 5-week high-amplitude vibration training caused a significant improvement in tPA (P = 0.028), it may be postulated that the vascular shear stress is more profound in high-amplitude vibration training. Skeletal muscle is known to be a release site of tPA, suggesting that skeletal muscle activation in WBV may stimulate the muscle to release tPA. Plasminogen is the inactive form of plasmin. It is converted to plasmin by tPA. tPA is the most abundant activator of fibrinolysis. During training, plasma concentration of PAI-1 is decreased which is partly due to its bounding to tPA. Previous studies have shown a decrease in PAI-1 by exercise.[35,36] An insignificant decrease has been shown in high-amplitude vibration training (P = 0.033).
Fibrinogen is one of the essential factors in the coagulation process. Fibrinogen is a high-molecular weight protein and is formed in the liver.[23] The results of our study showed a more profound, albeit insignificant decrease in concentration of fibrinogen in the all group, which may reflect conversion of fibrinogen to fibrin and further risk of clot formation. Furthermore, the nonsignificant results could be attributed to the small sample size in the different groups.
CONCLUSION
Whole body vibration training affects tPA and PAI-1 concentrations in healthy young men; and amplitude of vibrations seems an important variable in fibrinolytic factors.
Limitations of the study
The nonsignificant results could be attributed to the small sample size in the different groups. It seems that longer durations of training may have more profound effects on fibrinolytic factors.
AUTHORS CONTRIBUTION
FG contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. LH contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. LP contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MA contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work
ACKNOWLEDGMENT
The study with research project number (279/117) has performed under the supervision of Sport medicine federation of Iran and the costs have been paid by the federation.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gallistl S, Sudi KM, Cvirn G, Muntean W, Borkenstein M. Effects of short-term energy restriction and physical training on haemostatic risk factors for coronary heart disease in obese children and adolescents. Int J Obes Relat Metab Disord. 2001;25:529–32. doi: 10.1038/sj.ijo.0801573. [DOI] [PubMed] [Google Scholar]
- 2.Tofler GH, Massaro J, Levy D, Mittleman M, Sutherland P, Lipinska I, et al. Relation of the prothrombotic state to increasing age (from the Framingham Offspring Study) Am J Cardiol. 2005;96:1280–3. doi: 10.1016/j.amjcard.2005.06.072. [DOI] [PubMed] [Google Scholar]
- 3.van den Burg PJ, Hospers JE, Mosterd WL, Bouma BN, Huisveld IA. Aging, physical conditioning, and exercise-induced changes in hemostatic factors and reaction products. J Appl Physiol (1985) 2000;88:1558–64. doi: 10.1152/jappl.2000.88.5.1558. [DOI] [PubMed] [Google Scholar]
- 4.van den Burg PJ, Hospers JE, van Vliet M, Mosterd WL, Bouma BN, Huisveld IA. Changes in haemostatic factors and activation products after exercise in healthy subjects with different ages. Thromb Haemost. 1995;74:1457–64. [PubMed] [Google Scholar]
- 5.van den Burg PJ, Hospers JE, van Vliet M, Mosterd WL, Bouma BN, Huisveld IA. Effect of endurance training and seasonal fluctuation on coagulation and fibrinolysis in young sedentary men. J Appl Physiol (1985) 1997;82:613–20. doi: 10.1152/jappl.1997.82.2.613. [DOI] [PubMed] [Google Scholar]
- 6.El-Sayed MS, Sale C, Jones PG, Chester M. Blood hemostasis in exercise and training. Med Sci Sports Exerc. 2000;32:918–25. doi: 10.1097/00005768-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Robinson SD, Ludlam CA, Boon NA, Newby DE. Endothelial fibrinolytic capacity predicts future adverse cardiovascular events in patients with coronary heart disease. Arterioscler Thromb Vasc Biol. 2007;27:1651–6. doi: 10.1161/ATVBAHA.107.143248. [DOI] [PubMed] [Google Scholar]
- 8.El-Sayed MS, Lin X, Rattu AJ. Blood coagulation and fibrinolysis at rest and in response to maximal exercise before and after a physical conditioning programme. Blood Coagul Fibrinolysis. 1995;6:747–52. doi: 10.1097/00001721-199512000-00009. [DOI] [PubMed] [Google Scholar]
- 9.Koenig W, Ernst E. Exercise and thrombosis. Coron Artery Dis. 2000;11:123–7. doi: 10.1097/00019501-200003000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Hilberg T, Prasa D, Stürzebecher J, Gläser D, Schneider K, Gabriel HH. Blood coagulation and fibrinolysis after extreme short-term exercise. Thromb Res. 2003;109:271–7. doi: 10.1016/s0049-3848(03)00283-4. [DOI] [PubMed] [Google Scholar]
- 11.Hilberg T, Gläser D, Reckhart C, Prasa D, Stürzebecher J, Gabriel HH. Blood coagulation and fibrinolysis after long-duration treadmill exercise controlled by individual anaerobic threshold. Eur J Appl Physiol. 2003;90:639–42. doi: 10.1007/s00421-003-0907-2. [DOI] [PubMed] [Google Scholar]
- 12.Cerneca F, Crocetti G, Gombacci A, Simeone R, Tamaro G, Mangiarotti MA. Variations in hemostatic parameters after near-maximum exercise and specific tests in athletes. J Sports Med Phys Fitness. 1999;39:31–6. [PubMed] [Google Scholar]
- 13.Coppola L, Grassia A, Coppola A, Tondi G, Peluso G, Mordente S, et al. Effects of a moderate-intensity aerobic program on blood viscosity, platelet aggregation and fibrinolytic balance in young and middle-aged sedentary subjects. Blood Coagul Fibrinolysis. 2004;15:31–7. doi: 10.1097/00001721-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Dufaux B, Order U, Liesen H. Effect of a short maximal physical exercise on coagulation, fibrinolysis, and complement system. Int J Sports Med. 1991;12(Suppl 1):S38–42. doi: 10.1055/s-2007-1024748. [DOI] [PubMed] [Google Scholar]
- 15.el-Sayed MS. Effects of high and low intensity aerobic conditioning programs on blood fibrinolysis and lipid profile. Blood Coagul Fibrinolysis. 1996;7:484–90. doi: 10.1097/00001721-199606000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, et al. AHA Science Advisory. Resistance exercise in individuals with and without cardiovascular disease: Benefits, rationale, safety, and prescription: An advisory from the Committee on Exercise, Rehabilitation, and Prevention, Council on Clinical Cardiology, American Heart Association; Position paper endorsed by the American College of Sports Medicine. Circulation. 2000;101:828–33. doi: 10.1161/01.cir.101.7.828. [DOI] [PubMed] [Google Scholar]
- 17.Montgomery HE, Clarkson P, Nwose OM, Mikailidis DP, Jagroop IA, Dollery C, et al. The acute rise in plasma fibrinogen concentration with exercise is influenced by the G-453-A polymorphism of the beta-fibrinogen gene. Arterioscler Thromb Vasc Biol. 1996;16:386–91. doi: 10.1161/01.atv.16.3.386. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale M, Wakeling J. Whole body vibration exercise: Are vibrations good for you? Br J Sports Med. 2005;39:585–9. doi: 10.1136/bjsm.2005.016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rehn B, Lidström J, Skoglund J, Lindström B. Effects on leg muscular performance from whole-body vibration exercise: A systematic review. Scand J Med Sci Sports. 2007;17:2–11. doi: 10.1111/j.1600-0838.2006.00578.x. [DOI] [PubMed] [Google Scholar]
- 20.Rittweger J, Schiessl H, Felsenberg D. Oxygen uptake during whole-body vibration exercise: Comparison with squatting as a slow voluntary movement. Eur J Appl Physiol. 2001;86:169–73. doi: 10.1007/s004210100511. [DOI] [PubMed] [Google Scholar]
- 21.Cesarman-Maus GH, Katherine A. Molecular mechanisms of fibrinolysis. Br J Hematol. 2005;3:307–21. doi: 10.1111/j.1365-2141.2005.05444.x. [DOI] [PubMed] [Google Scholar]
- 22.Fay WP, Parker AC, Condrey LR, Shapiro AD. Human plasminogen activator inhibitor-1 (PAI-1) deficiency: Characterization of a large kindred with a null mutation in the PAI-1 gene. Blood. 1997;90:204–8. [PubMed] [Google Scholar]
- 23.Hall JE, Guyton AC. Philadelphia: Saunders Elsevier; 2011. Guyton and Hall Textbook of Medical Physiology; pp. 453–4. [Google Scholar]
- 24.Womack CJ, Rasmussen JM, Vickers DG, Paton CM, Osmond PJ, Davis GL. Changes in fibrinolysis following exercise above and below lactate threshold. Thromb Res. 2006;118:263–8. doi: 10.1016/j.thromres.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 25.De Gail P, Lance JW, Neilson PD. Differential effects on tonic and phasic reflex mechanisms produced by vibration of muscles in man. J Neurol Neurosurg Psychiatry. 1966;29:1–11. doi: 10.1136/jnnp.29.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews PB. The reflex excitation of the soleus muscle of the decerebrate cat caused by vibbration applied to its tendon. J Physiol. 1966;184:450–72. doi: 10.1113/jphysiol.1966.sp007926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hagbarth K, Eklund G. Motor effects of ventiratory stimuli. In: Granit R, editor. Proceedings of 1st Symposium on Muscular Afferents and Motor Control. Stockholm: Almqvist and Wiksell; 1985. pp. 177–86. [Google Scholar]
- 28.Seidel H. Myoelectric reactions to ultra-low frequency and low-frequency whole body vibration. Eur J Appl Physiol Occup Physiol. 1988;57:558–62. doi: 10.1007/BF00418462. [DOI] [PubMed] [Google Scholar]
- 29.Cardinale M, Lim J. Electromyography activity of vastus lateralis muscle during whole-body vibrations of different frequencies. J Strength Cond Res. 2003;17:621–4. doi: 10.1519/1533-4287(2003)017<0621:eaovlm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 30.Kerschan-Schindl K, Grampp S, Henk C, Resch H, Preisinger E, Fialka-Moser V, et al. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin Physiol. 2001;21:377–82. doi: 10.1046/j.1365-2281.2001.00335.x. [DOI] [PubMed] [Google Scholar]
- 31.Boyle LJ, Nagelkirk PR. The effects of whole body vibration and exercise on fibrinolysis in men. Eur J Appl Physiol. 2010;110:1057–61. doi: 10.1007/s00421-010-1590-8. [DOI] [PubMed] [Google Scholar]
- 32.Lohman EB, 3rd, Petrofsky JS, Maloney-Hinds C, Betts-Schwab H, Thorpe D. The effect of whole body vibration on lower extremity skin blood flow in normal subjects. Med Sci Monit. 2007;13:CR71–6. [PubMed] [Google Scholar]
- 33.Maloney-Hinds C, Petrofsky JS, Zimmerman G. The effect of 30 Hz vs 50 Hz passive vibration and duration of vibration on skin blood flow in the arm. Med Sci Monit. 2008;14:CR112–6. [PubMed] [Google Scholar]
- 34.Rittweger J, Beller G, Felsenberg D. Acute physiological effects of exhaustive whole-body vibration exercise in man. Clin Physiol. 2000;20:134–42. doi: 10.1046/j.1365-2281.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 35.Dalia MK, Azza FE, Amal AW, Manal EA. Effect of different exercise intensities on blood coagulation and fibrinolysis in oral contraceptive women. Bull Fac Pharm Ther Cairo Univ. 2007;12:119–29. [Google Scholar]
- 36.Jahangard T, Torkaman G, Ghoosheh B, Hedayati M, Dibaj A. The effect of short-term aerobic training on coagulation and fibrinolytic factors in sedentary healthy postmenopausal women. Maturitas. 2009;64:223–7. doi: 10.1016/j.maturitas.2009.09.003. [DOI] [PubMed] [Google Scholar]


