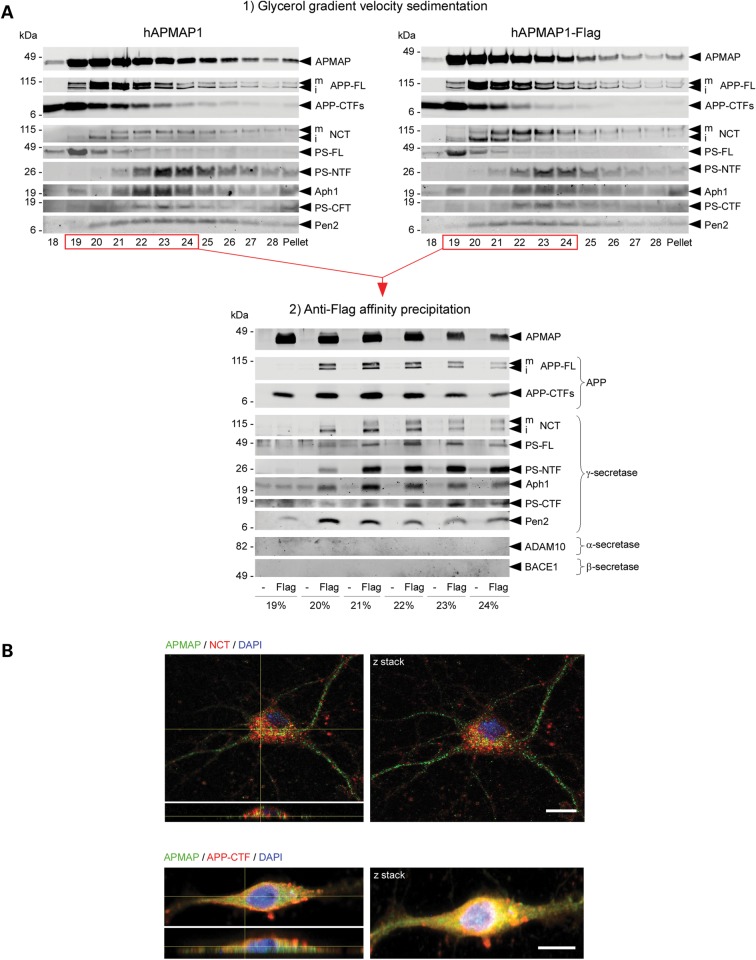
Figure 2.
APMAP interacts physically and co-localizes with γ-secretase, APP-FL and APP-CTFs. (A) Velocity co-sedimentation and co-immunoprecipitation of APMAP with the γ-secretase complex, APP-FL and APP-CTFs. Total membrane protein extracts from HEK-APPSwe cells transiently overexpressing hAPMAP1 or hAPMAP1-Flag were sedimented on an 18–28% glycerol gradient containing 0.1% CHAPSO. Each fraction was collected and analyzed by western blot for APMAP1, APP-FL, APP-CTFs and mature and immature γ-secretase (top panels). Next, proteins interacting with hAPMAP1-Flag (Flag) were affinity-precipitated in the fractions labeled in red with M2 anti-Flag affinity resin (lower panel). Untagged APMAP (hAPMAP1, also labeled ‘-’ in the figure) served as a control for the specific co-precipitation. (B) Immunohistochemical co-localization of APMAP (green) with the γ-secretase subunit Nicastrin (red, upper panel) or APP (red, lower panel) in 14 days in vitro mouse primary cortical neurons. Scale bar: 10 µm. Both confocal images (left panels) and Z-stack projections (right panels) are shown with a microscope objective magnification of 40×. For comparison, un-merged images for APMAP, NCT, APP-CTFs and DAPI are shown in Supplementary Material, Fig. S10. mNCT and iNCT, mature and immature Nicastrin.