Abstract
Cyanobacterial HCO3 - transporters BCT1, SbtA and BicA are important components of cyanobacterial CO2-concentration mechanisms. They also show potential in applications aimed at improving photosynthetic rates and yield when expressed in the chloroplasts of C3 crop species. The present study investigated the feasibility of using Escherichia coli to assess function of a range of SbtA and BicA transporters in a heterologous expression system, ultimately for selection of transporters suitable for chloroplast expression. Here, we demonstrate that six β-forms of SbtA are active in E. coli, although other tested bicarbonate transporters were inactive. The sbtA clones were derived from Synechococcus sp. WH5701, Cyanobium sp. PCC7001, Cyanobium sp. PCC6307, Synechococcus elongatus PCC7942, Synechocystis sp. PCC6803, and Synechococcus sp. PCC7002. The six SbtA homologs varied in bicarbonate uptake kinetics and sodium requirements in E. coli. In particular, SbtA from PCC7001 showed the lowest uptake affinity and highest flux rate and was capable of increasing the internal inorganic carbon pool by more than 8 mM relative to controls lacking transporters. Importantly, we were able to show that the SbtB protein (encoded by a companion gene near sbtA) binds to SbtA and suppresses bicarbonate uptake function of SbtA in E. coli, suggesting a role in post-translational regulation of SbtA, possibly as an inhibitor in the dark. This study established E. coli as a heterologous expression and analysis system for HCO3 - transporters from cyanobacteria, and identified several SbtA transporters as useful for expression in the chloroplast inner envelope membranes of higher plants.
Introduction
Due to projections in global population growth, there have been calls for a near doubling of global food production by 2050 [1], [2]. To meet this demand, scientists are exploring numerous genetic engineering strategies to increase crop yields by improving photosynthesis, particularly by increasing photosynthetic rates and/or water-use efficiency in crops. In C3 crop plants the current level of atmospheric CO2 is sub-optimal for maximal photosynthetic performance, with the competing oxygenase reaction of the primary carboxylase, Rubisco, accounting for around 30% of theoretical loss to photosynthetic CO2 fixation capacity [3]. Field studies have shown that elevated CO2 levels can increase photosynthetic rates and crop yields [4], [5]. This suggests that strategies aimed at raising CO2 levels in the chloroplast may be a useful approach. Recently, one multiple-stage approach to raise CO2 levels in the chloroplasts of crops was proposed based on the CO2-concentrating mechanism (CCM) components of photosynthetic bacteria cyanobacteria [6]–[8]. Two key features of the cyanobacterial CCM are the use of active transport systems for uptake of inorganic carbon (Ci, including CO2 and HCO3 -) and the elevation of CO2 levels within unique protein micro-compartments, called carboxysomes, which are packed with the Rubisco enzyme [9], [10].
Cyanobacterial Ci uptake systems in model species such as Synechococcus elongatus PCC7942 and Synechocystis sp. PCC6803 are composed of two known active CO2 uptake systems and up to three known HCO3 - transporters. They have different substrate affinities, maximal rates and energisation, which may provide different advantages for expression in C3 chloroplasts. Three HCO3 - transporters, including BCT1, SbtA and BicA, have been identified so far [6]. Among these transporters, BCT1 is a four-subunit ATP-binding cassette (ABC) transporter while SbtA and BicA are both single subunit transporters. SbtA and BicA have been initially chosen as candidates to be expressed in crops because they are both encoded by a single gene and therefore much easier to manipulate.
The SbtA transporter is a high affinity and low flux rate HCO3 - transporter, for example, SbtA affinity determined in Synechococcus PCC7002 has a Km[HCO3 -] of about 2 µM [11]. SbtA is Na+-dependent, requiring about 1 mM Na+ for half-maximal HCO3 - transport activity [12]. The gene encoding SbtA, sbtA, is inducible under limiting Ci conditions. SbtA has 10 transmembrane domains, in a 5+5 inverted orientation with the N- and C-termini extra cellular and the two halves of the transporter are separated by an intracellular loop of variable size [9]. Curiously, a gene, sbtB, encoding a small soluble protein (SbtB) is found to exist in the same operon as sbtA in some cyanobacterial species and nearby in others [13]. The sbtB gene is also expressed under Ci-limited conditions in Synechocystis sp. PCC6803 and Synechococcus elongatus PCC7942 [14], [15]. The co-occurrence suggests that SbtB may be functionally related to SbtA, possibly as a regulator, but this has not yet been investigated.
The BicA transporter can support a high photosynthetic flux rate, although it has a relatively low transport affinity with a Km [HCO3 -] of 75–350 µM [11]. BicA is also Na+-dependent and requires, similar to SbtA, about 1 mM Na+ for half-maximal transport activity [11]. BicA is predicted to be a single subunit transporter and belongs to the SulP/SLC26A protein family. Topology mapping and threading to a known crystal structure of related proteins strongly support 14 transmembrane domains with the N- and C- termini in the cytoplasm [16].
Although both transporters seem good candidates for expression in chloroplasts, a complicating fact is that both undergo some form of post-translational regulation because Ci uptake in cyanobacteria appears to be inactive in the dark [8]. Therefore it is unclear whether these transporters will be active when expressed in crops. In fact, recently, BicA expressed in the tobacco chloroplasts appeared to be inactive [17]. A better understanding of their regulation may allow manipulation of their regulatory systems or co-expression of activators, overcoming possible problems with inhibition. To this end we needed to develop a heterologous system for selection and characterisation of transporters which are active in non-cyanobacterial environment.
Both SbtA and BicA are widely distributed within cyanobacterial species, resulting in the availability of many different homologs to screen for ease of expression and regulatory properties [13]. Cyanobacteria are divided into two phylogenetic groups based on their Rubisco and carboxysomes phylogenies, referred to as α-cyanobacteria (largely oceanic) and β-cyanobacteria (freshwater, estuarine), based on their Rubisco and carboxysomes phylogenies [18]. In general, α-cyanobacteria have only a minimal CCM and possess fewer constitutively expressed Ci transporters while the β-cyanobacteria have much more diverse range of Ci transporters [13], [18]. In addition to generally defined α- and β- cyanobacteria, some strains of α-cyanobacteria (typically Cyanobium strains) have been classified as transitional strains since they have moved to freshwater estuarine environments and gained genes, including Ci uptake systems, from β-cyanobacteria, probably through horizontal gene transfer [13]. This conclusion was supported by the similarity in kinetic response of external Ci by the transitional strain, Cyanobium spp. PCC7001, to β-cyanobacteria Synechococcus elongatus PCC7942 [19].
There also exist sequence differences within each transporter family that correlate with the cyanobacterial classification. For example, the loop connecting helix 5 and 6 of SbtA in the transitional strains is much shorter than the loop in β-cyanobacteria [9]. It has been suggested a partial deletion in this region may have occurred at the time of horizontal gene transfer [13]. The functional importance of the helix 5/6 loop remains to be determined, but it may have a regulatory role or a link with HCO3 - transport affinity. To date, transporters shown to have HCO3 - uptake activity are mostly from α-cyanobacteria, including SbtA from Synechocystis sp. PCC6803 [12] and Synechococcus sp. PCC7002 [11], BicA from Synechococcus sp. PCC7002 [11] and BCT1 from Synechococcus elongatus PCC7942 [20]; the only α-cyanobacterial HCO3 - transporter analysed was BicA from Synechococcus WH8102 [11].
The aim of the present study was to investigate the expression of a range of SbtA and BicA transporters in E. coli for further characterisation. E. coli is considered a good candidate for study of cyanobacterial HCO3 - transporters for two reasons. First, there already exists a high CO2-dependent E. coli mutant (EDCM636) that may allow positive selection of HCO3 - transporters. E. coli possesses two carbonic anhydrases, Can and CynT [21]. CynT is normally not expressed, so the can gene knockout lacks carbonic anhydrase (CA) activity, and E. coli can grow in high CO2 but not in normal air due to lack of internal HCO3 - supply [21]. Since HCO3 - is required for anaplerotic metabolism, expression of an active HCO3 - transporter should theoretically restore growth of CA-deficient E. coli in air and therefore could allow positive screening of HCO3 - transporters. Second, topology mapping of BicA and SbtA [9], [22] has determined that both full length transporters are expressed in the E. coli plasma membrane, although uptake function was not previously examined. A potential drawback of utilising E. coli as a heterologous system for quantitative HCO3 - transport analyses is that the CO2 generated by cell respiration may introduce errors in determining the kinetics of 14C-HCO3 - uptake by these transporters which is further discussed in the context of the results presented.
In this paper, we demonstrate that six SbtA homologs are active in our E. coli expression system, three from the transitional strains, Synechococcus sp. WH5701 (SbtA5701), Cyanobium spp. PCC7001 (SbtA7001) and Cyanobium sp. PCC6307 (SbtA6307) and three from β-cyanobacteria, Synechococcus elongatus PCC7942 (SbtA7942), Synechocystis sp. PCC6803 (SbtA6803), and Synechococcus sp. PCC7002 (SbtA7002). Importantly, this is also the first experimental evidence that four SbtA homologs, SbtA7942, SbtA6307, SbtA5701 and SbtA7001, are in fact functional HCO3 - transporters. Additionally, our analyses begin to define a role for SbtB as a post-translational regulator of SbtA, potentially via direct interaction of these two proteins.
Results
Screening for putative HCO3 - transporters that are functional in E. coli
A number of putative HCO3 - transporters were screened in E. coli for HCO3 - uptake activity (Table 1). The respective cDNA sequence of each transporter was cloned into the pSE2 vector as illustrated in Fig. 1. The threshold for significant activity was set at a 2-fold increase in HCO3 - uptake compared to an empty pSE2 vector (negative control) at pH 8. All putative transporters belong to families where some members had been proven to have HCO3 - transport activity, including BCT1 [20], BicA7002 [11], SbtA6803 [12], and SbtA7002 [11]. We also included uncharacterised SbtA and BicA homologs in our analysis. Note that SbtA proteins from oceanic α-cyanobacteria were excluded since initial testing of SbtA from Prochlorococcus MED4 (CCMP1986) in our cyanobacterial expression system [11] had revealed no detectable transport activity. Two screening methods were used: HCO3 - uptake experiments and complementation of the CA-deficient strain EDCM636.
Table 1. HCO3 - transporters tested for function in E. coli.
| Transporter | Derivation Strain | HCO3 - Uptake | |||
| ATP-binding Cassette (ABC) Family | |||||
| BCT1 | Synechococcus spp. PCC7942 | No | |||
| Sulphate Permease (SulP) Family | |||||
| BicA7002 | Synechococcus spp. PCC 7002 | No | |||
| BicA5701 | Synechococcus spp. WH5701 | No | |||
| BicA1 7001 | Cyanobium spp. PCC7001 | No | |||
| BicA2 7001 | Cyanobium spp. PCC7001 | No | |||
| Vibrio SulP | Vibrio parahaemolyticus | No | |||
| Sodium Dependent Bicarbonate Transporter (SBT) Family | |||||
| SbtA7001 (SbtA1) | Cyanobium spp. PCC7001 | Yes | |||
| SbtA7942 | Synechococcus spp. PCC7942 | Yes | |||
| SbtA6803 | Synechocystis spp. PCC6803 | Yes | |||
| SbtA7002 | Synechococcus spp. PCC 7002 | Yes | |||
| SbtA6307 (SbtA1) | Cyanobium spp. PCC6307 | Yes | |||
| SbtA5701 (SbtA1) | Synechococcus spp. WH5701 | Yes | |||
| Labrenzia SbtA-like | Labrenzia alexandrii DFL-11 | No | |||
A number of known and putative HCO3 - transporters were tested in E. coli DH5α for potential H14CO3 - uptake activity measured by the silicon oil centrifugation-filtration assay.
Figure 1. Construct designs involved in characterisation of SbtA transporters.
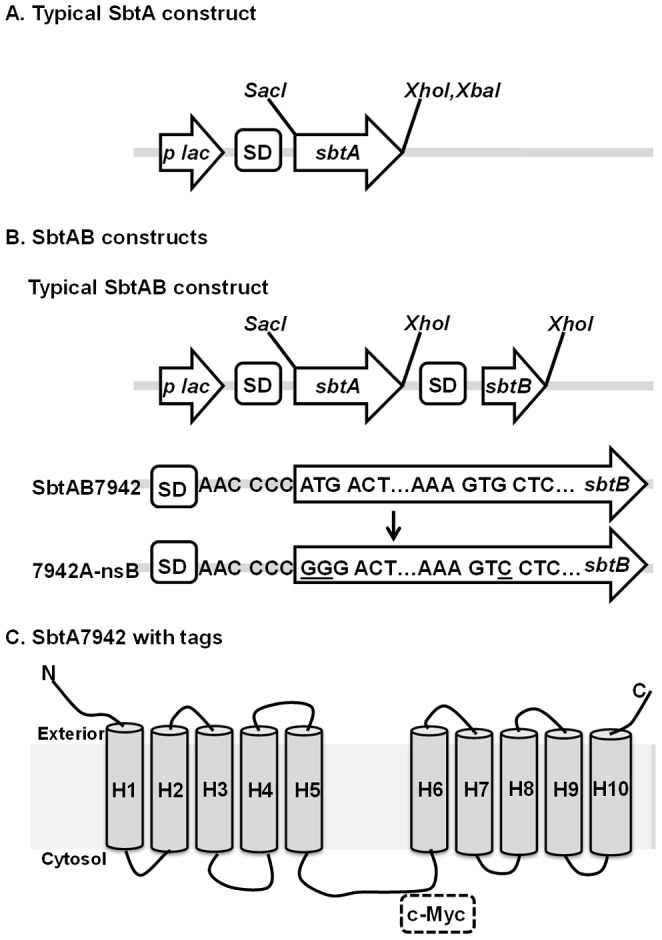
In all constructs, expression of target proteins was driven by the lac promoter on plasmid pSE2. A. Schematic of typical SbtA constructs shown in Table 4. B. Schematic of typical SbtAB constructs shown in Table 4. In order to generate 7942AnsB, the start codon of sbtB (ATG) in SbtAB7942 construct was replaced with (GGG) to form a SmaI site and a later GTG -valine 41 bp downstream of the start codon was replaced with GTC-valine. In this way, expression of sbtB is completely abolished in 7942AnsB. C. Illustrated location for the c-Myc tag in 7942AMyc. SD, Shine-Dalgarno sequence.
Expression of all SbtA homologs, with the exception of the Labrenzia SbtA-like transporter, facilitated enhanced HCO3 - uptake in E. coli while none of the other potential bicarbonate transporter appeared to increase HCO3 - uptake of E. coli (Table 1), strongly suggesting that all tested cyanobacterial SbtA homologs were able to transport HCO3 - and to function in the heterologous E. coli system.
Consistent with the uptake data, growth of EDCM636 in air was complemented only by expression of cyanobacterial SbtA homologs. There was no obvious difference in the growth of EDCM636 in the presence of kinetically different SbtA homologs, suggesting that they were all capable of supplying enough HCO3 - for cell growth at room temperature (Fig. 2).
Figure 2. Complementation of the EDCM636 mutant by expression of various SbtA clones.
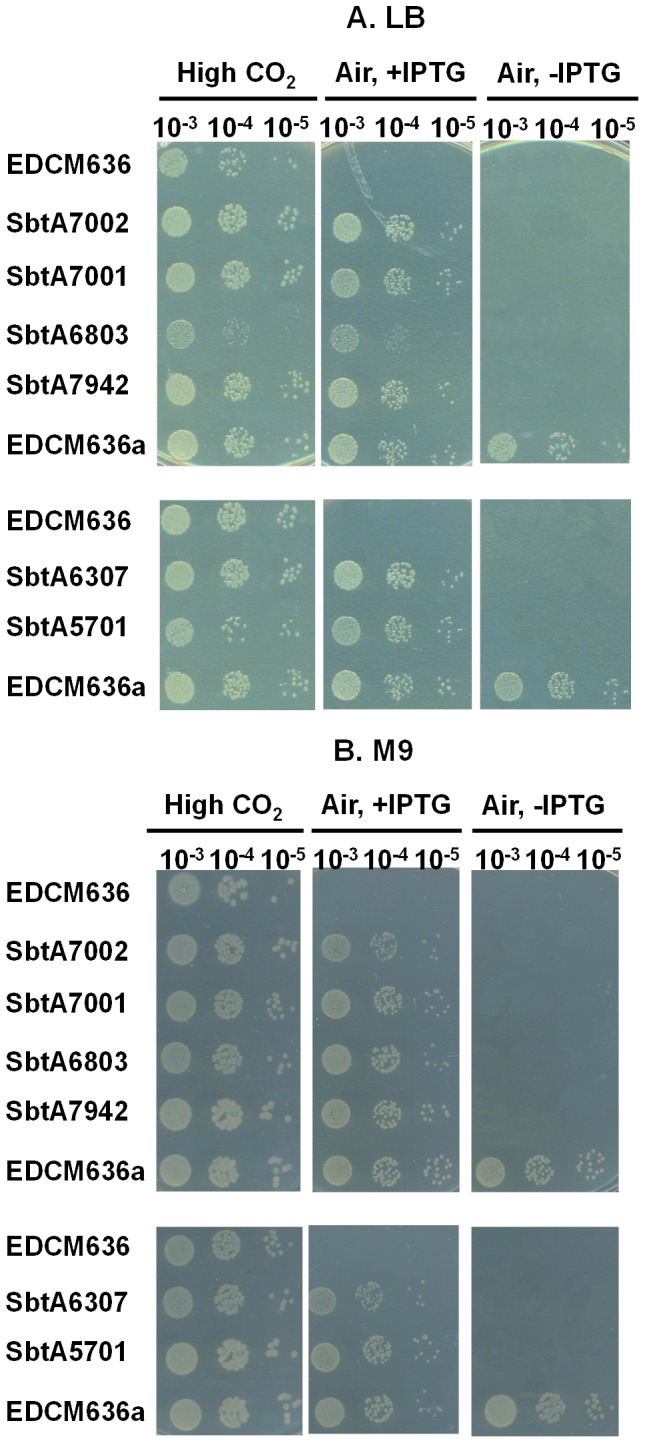
Complementation of CA-deficient strain EDCM636 expressing one of the six SbtA clones, or empty vector (pSE2) as a control. A strain with empty pSE2, EDCM636a, was selected for expressed CA. EDCM636a was used as a positive control. The top panel was for growth in LB media; bottom panel was for growth in M9 media.
Sodium dependency of HCO3 - uptake by SbtA homologs
As Synechocystis PCC6803 SbtA is Na+ dependent [12], we expected that this would also be the case for other SbtA homologs. Uptake activities of all SbtA species were stimulated by addition of NaCl, but not by comparable addition of KCl (S1 Fig.), indicating that these SbtA homologs are Na+ dependent in E. coli. The sodium dependence of each transporter was characterised in detail, ensuring other kinetic properties were analysed without Na+ limitation.
SbtA7942 and SbtA6307 required less Na+ compared to the others, with 1.5 mM and 0.8 mM Na+ for half maximal activities, respectively (Fig. 3 and Table 2). SbtA6803, SbtA5701 and SbtA7001 had intermediate requirements for Na+ and needed 3 to 5 mM Na+ to achieve half maximal HCO3 - uptake rates. The SbtA from a coastal marine species, SbtA7002, had the largest Na+ requirement of about 15 mM Na+ half maximal HCO3 - uptake rates. To some extent, the Na+ requirements for expressed SbtA clones were related to preferred habitat ranges [6] of the source species of each clone, with freshwater strains (PCC7942, PCC6803, PCC6307) and freshwater/estuarine strains (WH5701, PCC7001) having lower half-requirements than the marine/euryhaline strain, Synechococcus PCC7002. All SbtA transporters were saturated by 50 mM NaCl.
Figure 3. Sodium dependency of HCO3 - uptake due to expression of various SbtA clones.
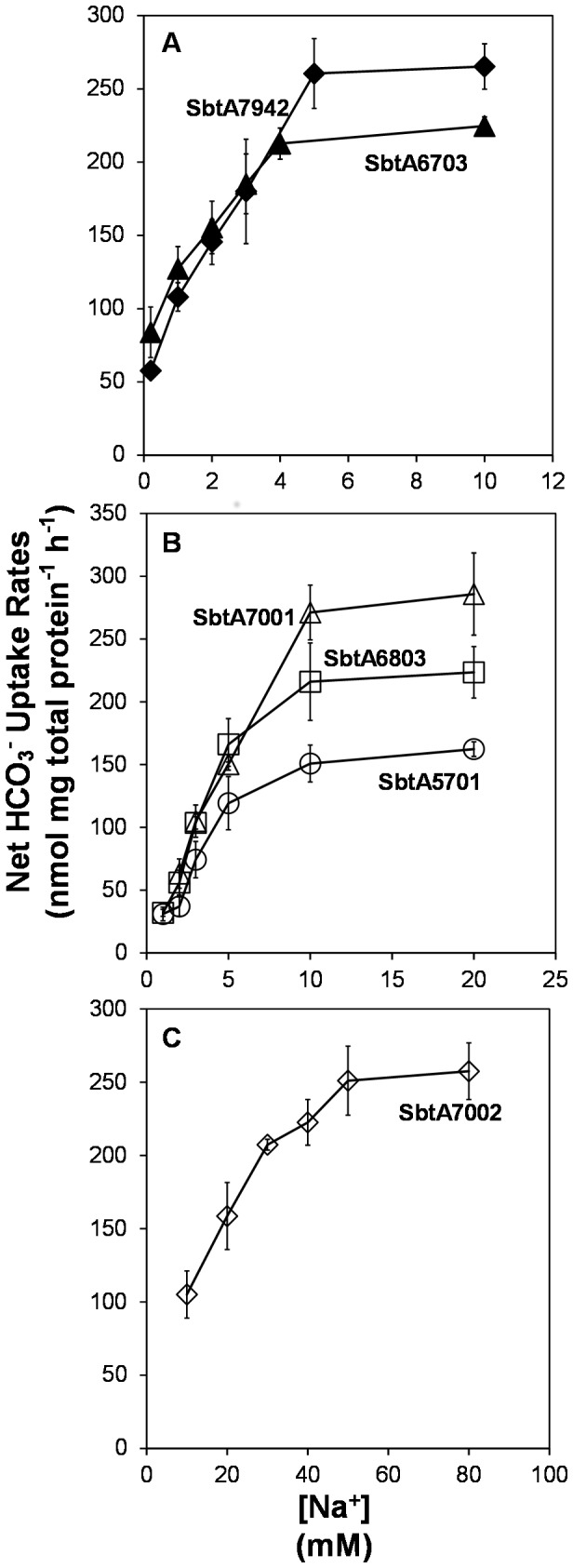
Cells were spun down and washed twice with CO2-free uptake buffer (22 mM potassium phosphate buffer, 20 mM pH 8 Bis-Tris-Propane-HCl pH 8). Additional NaCl was added to cells at various concentrations prior to uptake experiments. Net uptake rates were calculated by subtracting data of pSE2 empty (18–35 nmol mg total protein−1 h−1) from raw data of each transporter. Values in the figure are means ± SD (n = 6). SbtA7942, black diamond; SbtA6703, black triangle; SbtA6803, white square; SbtA5701, white circle; SbtA7001, white triangle and SbtA7002, white diamond.
Table 2. Sodium concentration required for half maximal and maximal activities of SbtA H14CO3 - uptake activity.
| SbtA clone | Na+ requirement for half maximal activity (mM) | Na+ requirement for maximal activity (mM) |
| SbtA7942 | 1.5 | 5 |
| SbtA6803 | 3 | 10 |
| SbtA7002 | 15 | 50 |
| SbtA6307 | 0.8 | 5 |
| SbtA5701 | 3.5 | 10 |
| SbtA7001 | 5 | 10 |
Values were derived from the curves in Fig. 3.
Affinity estimations and maximal HCO3 - uptake rates of SbtAs
Accurate determination of Km[HCO3 -] of high affinity SbtA transporters was difficult in E. coli because of CO2 generated from cell respiration which altered the effective unlabelled HCO3 - concentration. Despite all precautions (see material and Methods), around 80 µM Ci was generated due to cell respiration, as determined by mass spectrometer analysis. Most of the respiratory CO2 is present as HCO3 - in the buffer at alkaline pH. This source of HCO3 - increases the concentration of total HCO3 - and consequently reduces 14C specific activities (CPM nmol-1). Taking this into account resulted in the transformation of an initial raw Michaelis-Menten-like curve into a roughly flat line for SbtA7942 (Fig. 4A). This suggested that even the lowest Ci concentration was well above the true Km[HCO3 -]. Unfortunately, this was case for all the other SbtA transporters except for SbtA7001. It can only be concluded that the Km[HCO3 -] of SbtA7942, SbtA6803, SbtA7002, SbtA6307 and SbtA5701 were under 100 µM (20 µM injected HCO3 - plus ∼80 µM respiratory HCO3 -). SbtA7001, however, appears to have lower affinity and its Km[HCO3 -] was calculated to be 189 µM (Fig. 4B).
Figure 4. HCO3 - uptake of SbtA7942 and SbtA7001 against changes at external HCO3 - levels.
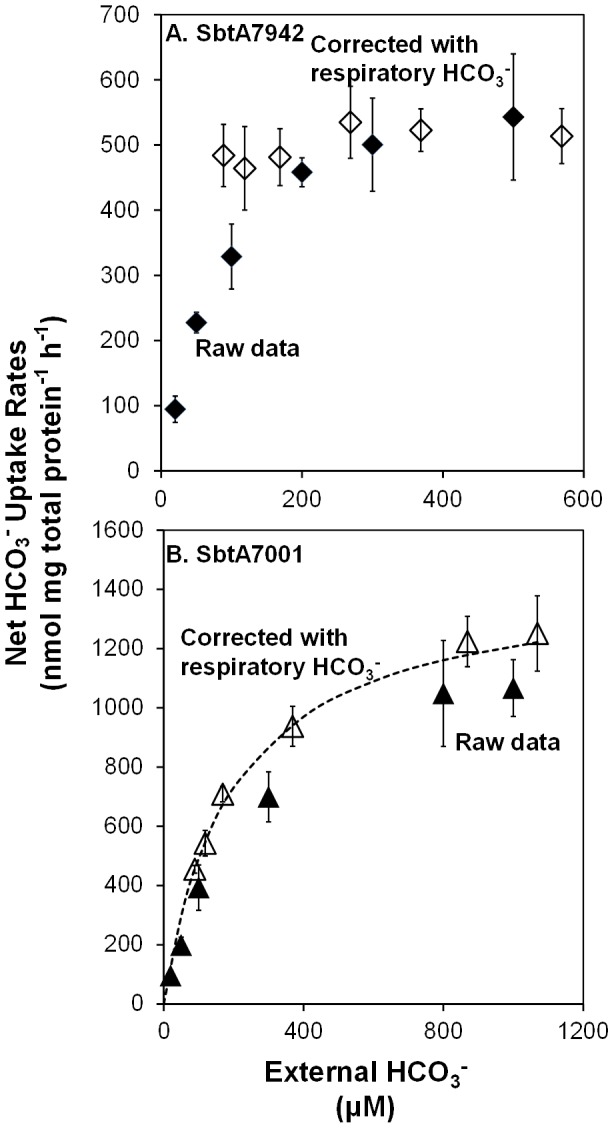
Uptake was measured as described in Materials and Methods. Respiratory HCO3 - levels were measured with MIMS allowing a correction for dilution of 14C-HCO3 - specific activity. Values in the figures are means ± SD (n = 6). A. HCO3 - uptake of SbtA7942. SbtA7942 Corrected (white diamond) uptake rates were calculated by subtraction of respiratory carbon from the SbtA7942 Raw data (black diamond). Six concentrations of Ci (20 to 500 µM) were injected to pSE2 or SbtA7942 cells. Raw uptake rates for pSE2 control were 6 to 199 nmol mg−1 h−1 and were corrected to 98 to 609 nmol mg−1 h−1. B. HCO3 - uptake of SbtA7001. SbtA7001 Corrected uptake rates (white triangle) were calculated by subtraction of respiratory Ci from the SbtA7001 Raw data (black triangle). Six concentrations of Ci (20 to 1000 µM) were injected to pSE2 or SbtA7001 cells. Raw uptake rates for pSE2 control were 9 to 294 nmol mg−1 h−1 and were corrected to 30 to 383 nmol mg−1 h−1. The theoretical Michaelis-Menten curve (Broken line) was calculated from SbtA7001 Corrected data (R2 = 0.9031).
Maximal uptake rates for HCO3 - of the different SbtAs can still be readily determined when respiratory CO2 was taken into account (Table 3). SbtA7001 showed a maximal uptake rate for HCO3 - at over 1200 nmol mg total protein−1 h−1. SbtA5701 and SbtA6307 had the lowest maximal activity and were able to transport HCO3 - at 200 nmol and 400 nmol mg total protein−1 h−1, respectively. SbtA7942, SbtA6803 and SbtA7002 had intermediate maximal HCO3 - uptake rates, ranging from 500 to 800 nmol mg total protein−1 h−1. For the BicA bicarbonate transporter a correlation between Vmax and Km[HCO3 -] has been observed for three BicA forms [11], and certainly SbtA7001 has the highest Vmax and Km, but the lack of precise Km data for the other SbtA forms makes full analysis premature at this point in time.
Table 3. Kinetics for HCO3 - uptake properties of SbtA homologs expressed in E. coli.
| SbtA clone | Km[HCO3 -] (µM) | Maximal HCO3 - uptake rates (nmol mg total protein−1 h−1) | Corrected HCO3 - uptake rates based on relative protein abundance |
| SbtA7942 | <100 | 559±47 | 559 |
| SbtA6803 | <100 | 756±14 | 1844 |
| SbtA7002 | <100 | 519±50 | 1573 |
| SbtA6307 | <100 | 390±56 | 1000 |
| SbtA5701 | <100 | 218±19 | 1816 |
| SbtA7001 | 189 | 1238±127 | 4585 |
Data were corrected with respiratory Ci. Maximal HCO3 - uptake rates were calculated from maximal Ci uptake, assuming that 98.1% Ci was HCO3 - at pH 8. Data were presented as mean ± SD (n = 6). Details of relative abundance of SbtA homologs were included in the Results section. Estimated relative abundances were based on Fig. 5: SbtA7942 (100%), SbtA6803 (41%), SbtA7002 (33%), SbtA 7001 (27%), SbtA5701 (12%) and SbtA6307 (39%).
Relative abundance of SbtAs in E. coli
In order to compare relative expression levels of the different SbtA proteins in E. coli, immunodetection was used to investigate abundance of all SbtA proteins in membrane-enriched fractions. A polyclonal anti-SbtA antibody cross-reacting very specifically with all SbtA proteins was used in this study. A single band at a molecular mass roughly 8–10 kD lower than predicted was observed for SbtA7002, SbtA7001, SbtA5701 and SbtA6307 (Fig. 5). Note that aberrant molecular mass on SDS PAGE is a very common observation with highly hydrophobic membrane proteins [17], [23], [24]. Both SbtA7942 and SbtA6803 also showed an additional band at around double the expected molecular weight. This may be a dimeric form of SbtA that has not been entirely disrupted by ionic detergent and reducing reagents. As equal amounts of total protein were loaded on the gel, the relative amount of each SbtA protein could be estimated based on image pixel volumes relative to a dilution series of SbtA7942 ranging from 20 to100% total protein loaded. Estimated relative abundances were: SbtA6803 (41%), SbtA7002 (33%), SbtA 7001 (27%), SbtA5701 (12%) and SbtA6307 (39%).
Figure 5. Relative accumulation of SbtA proteins expressed in enriched E. coli membrane fractions by western blotting.
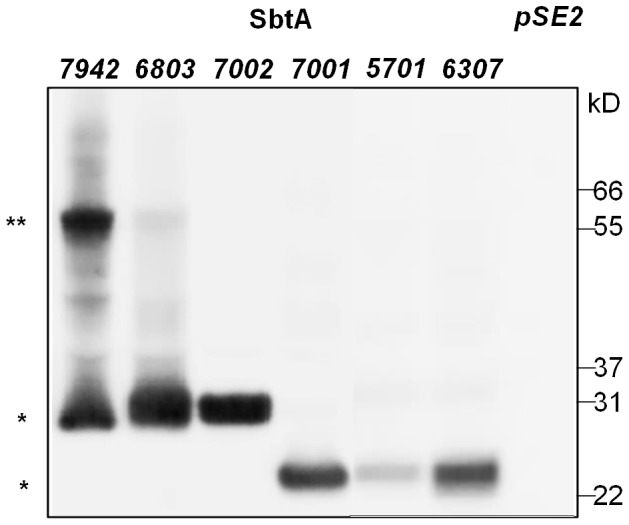
The respective sbtA genes were introduced on pSE2 plasmids under control of the IPTG inducible lac promoter. An empty vector (pSE2) served as negative control (right most lane). Gene expression was induced for 2.5 h with 1 mM IPTG. The membrane-enriched protein fractions were isolated, and 50 µg total protein per lane was separated by SDS-PAGE; bands detected by western blotting using the SbtA antibody. * = SbtA monomer; ** = possible dimer of SbtA.
Determination of relative HCO3 - uptake rates by correcting for the relative abundances of the various SbtA proteins allows estimation of the specific activity of each SbtA (Table 3). This suggests that there could be up to an eight-fold difference in Vmax, with SbtA7001 having the highest maximal HCO3 - uptake activity (4585 nmol mg total protein−1 h−1) and SbtA7942 the lowest (559 nmol mg total protein−1 h−1).
Effects of active SbtA on internal Ci pools
It is envisaged that expression of an active HCO3 - transporter in the chloroplasts of crop plants will need to elevate internal Ci levels [8] to subsequently improve photosynthetic rates. Therefore, we investigated the effects of expression of SbtA transporters on internal Ci pools of E. coli. The internal Ci pool (mM) was calculated based on corrected Ci uptake and using cell volumes determined as described in the Methods. Uptake of Ci was measured at 1000 µM injected Ci for SbtA7001 and at 500 µM for the other SbtA transporters to ensure maximal uptake rates for each SbtA transporter were achieved. Data were then corrected for respiratory CO2.
Internal Ci pool sizes were significantly increased in the presence of active SbtA transporters (Fig. 6). Expression of SbtA7001 led to the most significant increase, of about 8-fold increase compared to pSE2 only control, while the presence of SbtA5701 resulted in the smallest increase of only 2-fold. This equates to an increase in the internal Ci pool by more than 8 mM for E. coli expressing SbtA7001 and by 3–6 mM for the other SbtA transporters relative to controls without expressed transporters.
Figure 6. Internal Ci pool sizes of E. coli cells with the empty vector or various sbtA clones. The expression vector used was the pSE2 vector.
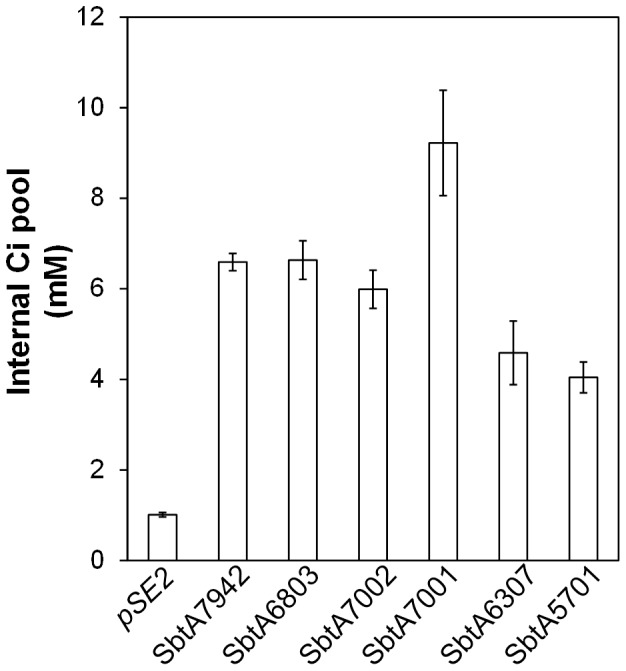
Internal Ci pools (mM) were calculated from maximum Ci uptake and the cell volume of each strain. Ci uptakes were measured in the presence of 500 µM injected H14CO3 - (except SbtA7001 which was 1000 µM) and corrected for respiratory Ci. Data as means ± SD (n = 6). The pools size of cells with each SbtA transporter was significantly different from the pool size of cells with the empty pSE2 vector as determined with the Welch's T-test (all p<0.02).
The role of SbtB in the regulation of SbtA uptake activity
The role of SbtB has not yet been determined but the co-location of the sbtB gene in, or near, the same operon as the sbtA gene suggests a potential role as a regulator of SbtA uptake activity or transcriptional expression. To investigate effects of SbtB on uptake activity of SbtA, five dicistronic sbtAB gene pairs were co-expressed from the lac promoter in E. coli (Fig. 1). All plasmid constructs lacked endogenous promoters for sbtA and/or sbtB genes to rule out the possibility of transcriptional control of SbtB on sbtA transcription. These five pairs were from cyanobacterial strains Synechococcus elongatus PCC7942 (SbtAB7942), Synechocystis sp. PCC6803 (SbtAB6803), Cyanobium sp. PCC7001 (SbtAB7001), Synechococcus sp. WH5701 (SbtAB5701) and Cyanobium gracile PCC6307 (SbtAB6307). Interestingly, active HCO3 - uptake was eliminated when SbtA was co-expressed with SbtB for SbtAB7942, SbtAB6803, SbtAB7001 and SbtAB5701 (Fig. 7). This suggests that SbtB may act as an inhibitor of SbtA activity, potentially by binding to SbtA. SbtAB6307 was an exception, with no effect of SbtB on SbtA activity. To date, the reason for the lack of effect is unclear and needs to be investigated further to determine whether SbtB has a different role in this species or there is a problem with the expression of SbtB. When SbtB was not present the transporters all showed normal uptake of HCO3 - (Fig. 7).
Figure 7. HCO3 - uptake capacity assessed for five separate SbtAB pairs and 7942A-nsB.
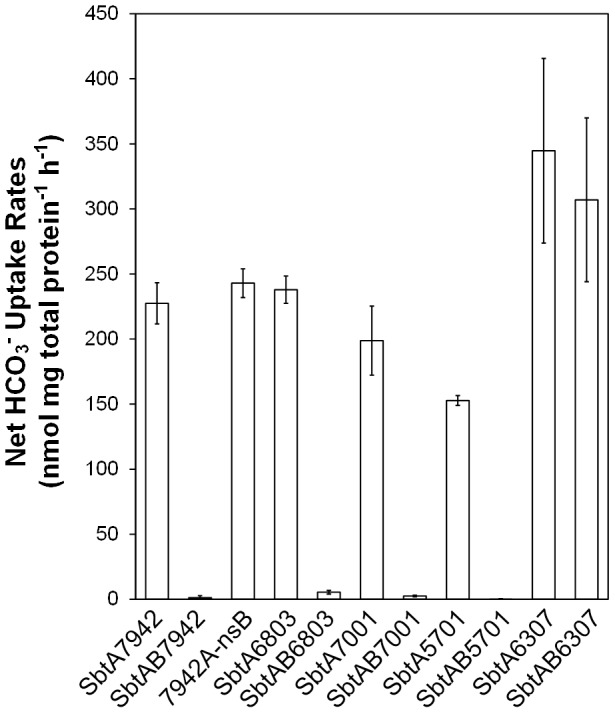
Uptake rates were calculated by subtracting data for the empty pSE2 control (∼22 nmol mg−1 h−1) from raw data of each strain. Data were not corrected with respiratory Ci as this is encompassed in the control value. Values in the figure are means ± SD (n = 6). The statistical significance of data was analysed with the Welch's T-test. The HCO3 - uptake rates of SbtA7942, SbtA6803, SbtA7001, SbtA7002 and SbtA5701 were significantly different with or without corresponding SbtB (all p<0.01). The HCO3 - uptake rates of SbtA6307 had no significant difference with or without SbtB6307 (p = 0.37). 7942A-nsB showed no significant difference in HCO3 - uptake rates to SbtA7942 (p = 0.59).
We investigated the possible regulatory role of SbtB further for SbtAB7942. Firstly, we generated a construct in which the start codon of SbtB was mutated from ATG to GGG, in construct 7942A-nsB (Fig. 1). This was designed to abolish translation of SbtB without impacting on translation of SbtA. This construct showed the same SbtA activity as the construct lacking SbtB (Fig. 7), indicating that inhibition is dependent on the presence of the SbtB protein.
Secondly, we investigated whether SbtA and SbtB interact. SbtA and SbtB were tagged with c-Myc and HA-His6, respectively, to allow immunochemical detection and affinity purification of SbtB (Table 4 and Fig. 1). The preliminary immunochemical detection of SbtA and SbtB with Western blotting showed that when both proteins were present, SbtA and SbtB were detected in the membrane fraction (S2 Fig.). However, when SbtB was expressed alone, it was detected in the soluble protein fraction (S2 Fig.). This result suggested a physical interaction between the two proteins.
Table 4. List of constructs involved in characterisation of SbtA transporters.
| pSE2 Construct names | Description | |
| SbtA constructs | SbtA7942 | sbtA from Synechococcus sp. PCC7942 |
| SbtA6803 | sbtA from Synechocystis sp. PCC6803 | |
| SbtA7001 | sbtA from Cyanobium sp. PCC7001 | |
| SbtA7002 | sbtA from Synechococcus sp. PCC 7002 | |
| SbtA6307 | sbtA from Cyanobium sp. PCC6307 | |
| SbtA5701 | sbtA from Synechococcus sp. WH5701 | |
| SbtAB constructs | SbtAB7942 | Artificial dicistronic clone for sbtA and sbtB, Synechococcus PCC7942 |
| SbtAB6803 | sbtA and sbtB6803 cloned as a natural dicistronic context | |
| SbtAB7001 | sbtA and sbtB7001 cloned as a natural dicistronic context | |
| SbtAB6307 | sbtA and sbtB6307 cloned as a natural dicistronic context | |
| SbtAB5701 | sbtA and sbtB5701 cloned as a natural dicistronic context | |
| 7942A-nsB | Start codon of sbtB altered (ATG to GGG) in SbtAB7942 | |
| Constructs for protein-protein interactions | 7942AMyc | c-Myc tag fused into loop 5/6 of sbtA7942 in SbtA7942 construct (at E203) |
| 7942AB-HAH6 | HA tag fused at the C-terminus of sbtB, based on SbtAB7942 construct | |
| 7942B-HAH6 | sbtB only generated from ABHAH67942 by PCR | |
| 7942AMBH | c-Myc tag fused into loop 5/6 of sbtA7942 based on the ABHAH6-7942 construct |
All constructs were based on pSE2 vector in which the expression of proteins was driven by the lac promoter. A c-Myc tag was fused to the 5/6 loop of SbtA7942 after position E203 of SbtA. The HA and His6 tags were fused to the C-terminus of SbtB7942. A schematic illustration of the constructs can be found in Fig. 1.
To confirm an interaction between SbtA and SbtB, we tested whether the two proteins co-purify from solubilised membrane-enriched fractions. Affinity chromatography was used to isolate His-tagged SbtB from the enriched membrane fraction in a construct expressing both SbtA and SbtB (7942AmBH, Table 4). Since only SbtB was tagged with His6, SbtA should not be detected unless it interacts with SbtB. Western blotting with the anti-c-Myc antibody showed that SbtA could be detected after purification (Fig. 8). No SbtA was detected when the affinity chromatography was repeated with the same construct lacking either SbtA or SbtB (Fig. 8). This strongly suggests that SbtA is purified because it interacts with SbtB, rather than through a non-specific interaction with the resin of the chromatography column.
Figure 8. Isolation of SbtA7942 by IMAC using SbtB-HAH6 as binding partner and detected by western blotting.
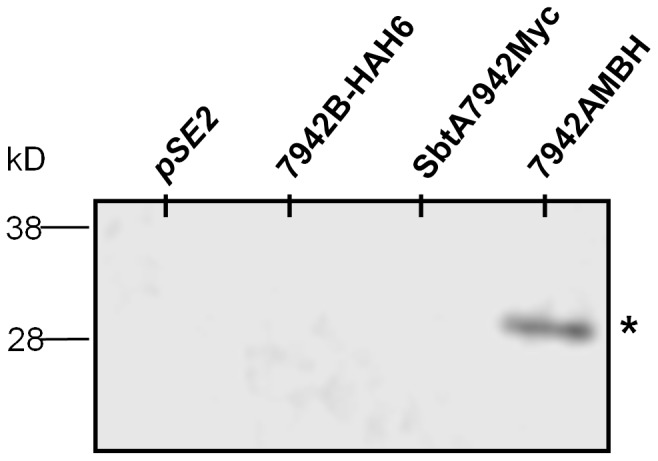
Gene expression was induced for 2.5 h with 1 mM IPTG. The membrane-enriched protein fractions of E. coli containing the empty pSE2, 7942AMyc and 7942AMBH and 7942B-HAH6 vector were used for IMAC isolation. A total of 40 µg total protein of IMAC elutes per lane was separated by SDS-PAGE and subjected to Western blotting. Proteins were detected with the Anti-c-Myc antibody.
Discussion
Characterisation of SbtA homologs in E. coli
In this study, we successfully demonstrated functional expression of a number of HCO3 - transporters and their homologs in E. coli. To our knowledge, this is first time this has been achieved in E. coli as a heterologous expression system. Six cyanobacterial SbtA homologs were shown to display HCO3 - uptake while members of the BicA and BCT1 families lacked any detectable uptake. It seems that at least for BicA7002, additional regulator(s) are required for its function in E. coli, because we were able to detect BicA in the enriched membrane fraction of E. coli (S3 Fig.). The experiments to detect BCT1 proteins were not conducted due to the lack of antibodies. In addition, this is the first experimental evidence that SbtA7942, SbtA7001, SbtA6307 and SbtA5701 homologs function as HCO3 - transporters. The latter three homologs were derived from transitional α-cyanobacteria of the Cyanobium clade but their sbtA genes are thought to have originated from β-cyanobacteria [13]. The other three homologs were derived from β-cyanobacteria. All six SbtA homologs were able to complement the EDCM636 CA-deficient mutant to restore growth at atmospheric CO2 levels (Fig. 2).
The six SbtA homologs chosen from β-cyanobacteria and transitional strains represent two groups showing minor protein sequence differences. Intriguingly, the most notable variation is in the size of the loop between helices 5 and 6 which separates the two homologous halves of the transporter [9], [13]. The loop is consistently 35–40 amino acids shorter in the SbtA proteins from transitional strains. We were interested in whether this correlated with any functional differences in Na+ requirements, maximal HCO3 - uptake rates or Km[HCO3 -]. However, the six SbtA homologs showed various Na+ requirements and HCO3 - uptake kinetics, unrelated to the sizes of the loop between helix 5 and 6, suggesting that the determinants of the properties we examined lie in other areas of difference.
Advantages and limitations of an E. coli system for analysis of cyanobacterial HCO3 - transporters
Our main objective was to study cyanobacterial HCO3 - transporters in a heterologous background where analysis was unlikely to be compromised by cyanobacterial regulatory factors involved in their activation/deactivation. Given its standard use in the laboratory for recombinant protein expression and molecular genetics as well as availability of a wide range of metabolic mutants, including carbonic anhydrase, E. coli was our system of choice. We developed two independent assays for function: a silicon oil centrifugation-filtration uptake assay and a complementation assay in E. coli. We were able to show that at least six members of the SbtA HCO3 - transporter family are expressed and functional in the absence of other cyanobacterial components and active photosynthesis. In other words, SbtA alone has the desirable property of being constitutively active. However, both assays have limitations that need to be taken into consideration. While the uptake assay allowed us to identify some kinetic parameters of the transporters, a drawback of this system is that we were not able to completely remove inorganic carbon, mainly CO2, generated by cell respiration. In contrast, respiratory Ci can be conveniently removed by a short period of photosynthetic CO2 fixation in a closed cuvette when using photosynthetic organisms for this type of analysis. In E. coli, the presence of respiratory Ci leads to dilution of radioactivity and an inability to provide cells with near-zero levels of Ci during uptake assays. The residual Ci concentration can be measured and corrected for, however, it remains impossible to measure uptake at Ci concentrations below the residual level. This is not problematic for kinetic measurements for HCO3 - transporters with medium to low affinity, as illustrated by the case of SbtA7001. However, existence of residual Ci hinders accurate determination of Km[HCO3 -] for high affinity HCO3 - transporters, as illustrated by the case of the remaining SbtA transporters. For example, in cyanobacteria, the SbtA7002 form is estimated to have a transport affinity as low as 2 µM [11].
Complementation of the CA-deficient E. coli strain, EDCM636, provides a convenient screen for function of individual transporters (Fig. 2). However, the strain reverts to wild type at a relatively high frequency as a consequence of the presence of a second wild type CA gene, cynT, that is not normally expressed, leading to selection for expression in any screening assay. In fact, about 12% of plated EDCM636 colonies regained CA activity and lost the need for high CO2 for growth [21]. In our case, we found that the occurrence of reversion could be reduced by taking extra precautions, for example using fresh cells from glycerol stocks. Nevertheless, this strategy would be unsuitable for large scale functional screening of HCO3 - transporters, for example, using cDNA or mutant libraries. In spite of these drawbacks, the two assays described here have been valuable in identifying and analysing HCO3 - transporters in a heterologous, non-photosynthetic system and will be useful for future investigations of SbtA structure and function.
Post-translational regulation of SbtA by SbtB
One novel and important finding of this study is that SbtB serves as a post-translational regulator of SbtA. Firstly, co-expression of SbtB inhibited HCO3 - uptake by SbtA in four out of five sbtAB expression pairs. The only exception was SbtAB6307 in which the uptake activity of SbtA6307 was not affected for unknown reasons, which could be as simple as lack of expression of the SbtB6307 protein. There are no SbtB antibodies available for testing this possibility. Generation of a tagged version of SbtB6307 with a HA or c-Myc epitope detectable by commercially available antibodies would be required, which is part of future investigations.
Secondly, the requirement for synthesis of the SbtB protein for inhibition and the fact that substantial amounts of SbtA protein accumulate in the presence of SbtB rules out regulation of expression at the transcriptional or translational level. Thirdly, there is a strong indication for direct protein-protein interaction between SbtA and SbtB. SbtA and SbtB was co-purified using a polyhistidine tag located on SbtB, indicating a strong physical interaction between SbtA and SbtB. In addition, immunodetection showed that SbtB7942 was only detectable in the plasma membrane when co-expressed with SbtA in E. coli (S2 Fig.). It is likely that in E. coli, SbtB regulates SbtA independently of secondary regulation processes in cyanobacteria. As such, it is interesting to speculate that in cyanobacteria SbtB might acts as a “curfew” protein to help inactivate SbtA in the dark, and that cyanobacteria would also have a mechanism to “unlock” SbtA in the light. SbtB shares low similarity (21% identity) in amino acid sequence with cyanobacterial PII proteins, and an unpublished crystal structure for SbtB from Anabaena (www.ncbi.nlm.nih.gov structure 3DFE) shows that β-SbtB has a very similar fold to PII (GlnB; structure 1QY7) from cyanobacteria. PII/GlnB proteins form trimers, are widely distributed in many bacteria, and are key regulators of nitrogen metabolism. This occurs through binding of effector molecules, indicating nitrogen status such as oxo-glutarate and ADP and post-translational interactions with a range of proteins [25].
It is noteworthy that the trimeric AmtB ammonia channel from E. coli is regulated by the binding of the GlnK trimer (PII homolog), with AmtB being inactive for ammonia influx when GlnK is bound, and active when unbound, at high levels of oxo-glutarate, ATP and Mg2+ [26], [27]. AmtB-GlnK could therefore make a useful working model for analysis of SbtA-SbtB regulation, despite potential differences in effectors required. Furthermore, because SbtB crystallises as a trimer (see above), it seems sensible to postulate that SbtA functions as a trimer; data suggesting that SbtA6803 runs on native gels as a 160 kDa tetramer [15] could also potentially be re-interpreted as 156 kDa expected size (3 times 40 kDa for SbtA plus 3 times 12 kDa SbtB). Future investigation is required to better understand the mechanism of SbtA regulation by SbtB, its role in modulating HCO3 - uptake activity of SbtA, and whether SbtB could also be involved in other signalling pathways in a similar way to PII (GlnB).
SbtA candidates for expression in crop plants
One longer term goal of our research is to identify candidate HCO3 - transporters to be expressed in crops [7], [8]. This requires that the transporters are active in heterologous systems and have kinetic properties that are consistent with functional expression in the chloroplast. Several SbtA homologs we tested are good candidates, with the best able to increase the Ci pool inside E. coli cells by up to 9 mM. We tested HCO3 - transporters from the BicA homolog grouping and found that none was functional in E. coli under our experimental conditions. Whether this is due to a need for unidentified regulatory factors is not yet known. However, all members of the SbtA family were functional and also showed interesting variation in their kinetic characteristics, allowing selection for those with the most potential for chloroplast expression.
It is estimated that at least 250 µM HCO3 - is present in the C3 leaf cytosol under ambient air [28] and that 1 to 3 mM Na+ is present in the cytoplasm [29], as an inwardly directed Na+ gradient across the chloroplast inner membrane [30]. This could potentially provide a suitable environment for increased accumulation of Ci in the chloroplast due to expression of at least some of the SbtA homologs characterised here. The Km[HCO3 -] of all SbtAs tested was below the 250 µM HCO3 - present in the leaf cytosol. SbtA7942, SbtA6803, SbtA6307 and SbtA5701 may represent more suitable candidates to be expressed in crops because of their lower requirements for Na+, with SbtA7942 and SbtA6307 needing only 1.5 mM and 0.8 mM Na+ respectively for half maximal uptake (Table 2). We are currently investigating the suitability of SbtA for functional expression in C3 chloroplasts.
Materials and Methods
Bacterial strains and growth conditions
E. coli K12 strain DH5α (F– Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rK–, mK+) phoA supE44 λ– thi-1 gyrA96 relA1) was used routinely for cloning, storage of plasmids and general expression of membrane proteins. E. coli for screening of HCO3 - transporters was a CA-deficient strain EDCM636, which is derived from E. coli MG1655 (F- λ-ilvG-rfb-50 rph-1) harbouring a kanamycin resistance marker replacing a deletion of the CA encoding gene can (Δcan) [21]. EDCM636a, a strain with restored CA function, was specially selected to provide a positive control in dilution spotting assays (see below). A second control strain also contained an empty pSE2 vector. Genes encoding for membrane proteins were cloned into the pSE2 vector where their expression was driven by the IPTG-inducible lacZ promoter [31]. The pSE2 vector carries a spectinomycin resistance gene as selectable marker. The main plasmid constructs involved in the characterisation of SbtA transporters are listed in Table 4 and Fig. 1.
Luria–Bertani (LB) broth and LB agar were used for routine bacterial growth in liquid culture while shaking or on solid medium, respectively. Unless specified, cells were grown at 37°C. For dilution spotting assay, E. coli was cultured on LB agar or M9 minimal agar with 0.4% glycerol [32]. Where applicable, antibiotics were added to the following final concentrations: kanamycin at 50 µg ml−1 and spectinomycin at 100 µg ml−1.
Dilution spotting assay
E. coli strains were grown on LB agar plates overnight. For strain EDCM636, 0.1 mM sodium azide was added to plates to induce expression of cynT [21]. The next morning, cells were resuspended in MilliQ water to OD600 of 0.1, and then diluted to 10−3, 10−4 and 10−5. An aliquot of 10 µl of each dilution was pipetted onto LB agar containing 20 mM Epps-HCl pH 8 or M9 agar with 0.4% glycerol and 20 mM Epps-HCl pH 8 supplemented with the appropriate antibiotics. Protein expression was induced with IPTG at a final concentration of 0.2 mM. Plates were incubated at 24°C for 2 days (LB) and 6 days (M9).
Bicarbonate uptake measurements
Bacterial strains for HCO3 - transporter expression and functional analysis were pre-grown for 16 h in 3 ml LB broth with spectinomycin, inoculated into 10 ml LB-spectinomycin broth and grown for 1 h. A final concentration of 1 mM IPTG was added to induce transporter gene expression for 3 h unless stated otherwise. Optimisation experiments showed the level of expression increased for 4 h IPTG induction but declined subsequently (S4 Fig.). Cells were harvested by centrifugation at 9,000 g for 30 s and washed twice with CO2-free uptake buffer (22 mM potassium phosphate, 20 mM Bis-Tris-Propane-HCl pH 8 and 50 mM NaCl. Modified uptake buffers with varying concentrations of Na+ were used in experiments to determine Na+ dependency of HCO3 - uptake. To remove CO2, the buffer was bubbled with high purity N2 for 3 days. Immediately before each uptake assay, cell aliquots were spun down and resuspended in CO2-free uptake buffer to minimize the time for respiratory CO2 release into the buffer.
Inorganic carbon uptake was determined by the silicon oil centrifugation-filtration assay described previously [33]. A stock solution of radioactive NaH14CO3 in “cold” NaHCO3 (25 mM, 0.11 mCi ml−1 pH 9.5) was added to cells at a final concentration of 50 µM (additions of NaH14CO3 were varied for kinetic measurements), cells were mixed and 100 µl was aliquots were transferred to micro-centrifuge tubes containing 5 µl of “kill” solution (3 M NaOH, 50% methanol) overlaid with 50 µl silicon oil mixture (AR20:AR200 4∶3.5 v/v). Bicarbonate uptake was stopped after 30 s by centrifugation, which was the shortest time in which HCO3 - uptake reached saturation (S5 Fig.). Tubes were frozen instantaneously in liquid nitrogen for further processing.
The tips of micro-centrifuge tubes containing the cell pellet in “kill” solution were cut off, cell pellets resuspended in 300 µl 2 M NaOH in scintillation vials, and 3 ml scintillation fluid (Ultima Gold XR, PerkinElmer) was added before measuring 14C CPM in a Beckman-Coulter scintillation counter. The specific activity of NaH14CO3 stock solution was calculated from CPM of 1 µl in 200 µl 2 M NaOH. Respiratory CO2 contamination was determined from cells treated as for H14CO3 - uptake experiments except using non-radioactive uptake buffer. After cells were spun down the supernatant was immediately transferred to a new tube, stored frozen and total Ci in the supernatant was measured with a membrane inlet mass spectrometer [34]. HCO3 - uptake rates were calculated as 98.1% of the raw Ci uptake rates based on the pKa of CO2 to HCO3 - at pH 8, 24°C and the ionic strength of the assay buffer [35]. Total protein concentration of each sample was determined using a BCA protein assay kit (Pierce) according to the manufacturer's protocol with bovine serum albumin as a standard.
Cell volume measurements
Silicon oil centrifugation-filtration removes most excess buffer as cells are spun down through the silicon oil layer except for a thin water (buffer) shell that forms around each cell. To determine the true cell volume, the total of the cell space plus the water shell is estimated from tritiated (3H) water which can enter E. coli cells and outer space. The water shell is estimated from 14C-Inulin, which cannot enter E. coli cells [36]. Thus, cell volume can be calculated by subtracting the water shell volume from the total.
Silicon oil centrifugation-filtration assays were performed as described above except that tritium or 14C-inulin was added to cells at a final concentration of 0.3 µCi ml−1. The incubation time was 10 min for tritium and 30 s for 14C-inulin. After centrifugation, 1 µl of the supernatant in each tube was kept for determination of specific activities. Cell volume (µl) was calculated for 1 ml cells at OD600 = 1. Cells containing the pSE2 vector had a combined cell volume of 2 to 2.5 µl whereas cells expressing the SbtA PCC7942 protein had a combined cell volume of 1 to 1.5 µl (averaged from at least 3 biological replications).
Preparation of membrane-enriched protein fractions of E. coli
Cell cultures grown for 14 h in LB broth with spectinomycin were diluted 1∶3, and after 1 h cells were induced for 2.5 h with 1 mM IPTG (final concentration). Cells were washed twice in lysis buffer (100 mM NaCl, 10 mM MgCl2 and 25 mM Tris-HCl, pH 8.0). After one freeze and thaw cycle, cell pellets were resuspended in lysis buffer with 1.4% (v/v) protease inhibitor (PI) cocktail (Complete mini, Roche) and approx. 100 µL of 0.1 mm glass beads (Sigma, USA). Cells were disrupted in a Tissuelyzer (Retsch, Germany) shaking for 5 min at 30 Hz in 1.5 mL microfuge tubes. Cell debris was removed by centrifugation for 15 s at 14,000 g at 4°C and transfer of the supernatants to new tubes. Crude membranes were collected by centrifugation at 14,000 g at 4°C for 10 min. For immunodetection, the supernatant (soluble protein fraction) and the pellets (crude membrane fraction) were supplemented with sodium dodecyl sulfate (SDS) sample buffer to final concentrations of 62.5 mM Tris-HCl, pH 6.8, 4% (w/v) SDS, 1 mM dithiothreitol (DTT) and 10% glycerol. Both fractions were incubated at 70°C for 20 min. The crude membrane fraction was centrifuged at 14,000 g for 15 min to precipitate insolubles. The total protein concentration of soluble protein fraction and enriched membrane fraction was determined with a detergent compatible (DC) protein assay kit (BioRad). Bromophenol blue (2 µg ml−1 final) was added prior to analysis by SDS-PAGE.
Isolation of SbtA:SbtB complexes
For isolation of SbtA:SbtB-HA-H6 complexes a crude membrane fraction was prepared and resuspended in buffer A (50 mM Bis-Tris pH 6.0, 2 mM CaCl2, 1 mM DTT, 10% glycerol with PI), frozen in liquid nitrogen and stored at -20°C.
Immobilized metal affinity chromatography (IMAC) was used for protein purification adapted from the method for isolating the native E. coli respiratory Complex I [37]. The following steps were carried out at 4°C. In brief, dodecyl-β-D-maltoside (DDM) was added to the crude membrane fraction to a final concentration of 1.2% (w/v). Samples were gently mixed for 1 h and centrifuged in a bench-top micro-centrifuge at 14,000 g for 20 min. The supernatant was transferred to a new tube, gradually supplemented with NaCl to a final concentration of 200 mM and mixed with IMAC resin (Profinity IMAC Ni-charged resins, BioRad) equilibrated with buffer A. The mixture was incubated with gentle mixing for 1 h, loaded onto a gravity packed column, and then washed with 2 column volumes of wash buffer (buffer A, 200 mM NaCl, 0.1% DDM and 5 mM histidine). The proteins were eluted with buffer A containing 200 mM NaCl, 0.1% DDM and 200 mM histidine. The eluates were mixed with SDS sample buffer and the concentration of total protein content was determined as described above.
SDS-PAGE and western blotting
E. coli membrane and soluble protein fractions were separated by SDS-PAGE on 4-12% Bis-Tris protein gels (NuPAGE, Invitrogen, USA) as described by the manufacturer. The expression level of SbtA was detected immuno-chemically after transfer to PVDF membrane with a polyclonal antibody (Agrisera, Sweden) directed against a conserved epitope of SbtA proteins from many β-cyanobacteria (PTLRAGIPSANPSAY, S6 Fig.). Tagged proteins were detected with monoclonal antibodies against these epitopes, anti-c-Myc (against EQKLISEEDL) or anti-HA (against YPYDVPDYA) (Sigma, USA). Proteins were visualized by fluorescence detection with an alkaline phosphatase-conjugated secondary antibody and the AttoPhos detection system (Promega, USA) on a Versadoc imager (BioRad, USA). Dilution series of the crude membrane fraction of SbtA7942 were loaded onto SDS-PAGE gels to ensure that the amount of proteins in samples were in the linear range for semi-quantitative analyses using Quantity One software (BioRad, USA).
Supporting Information
The effect of KCl and NaCl on the HCO3- uptake rates by SbtAs. Cells were prepared as described in the Methods except that a modified CO2-free buffer with 1 mM NaCl was used. Five mM KCl or NaCl was added to cells before the uptake experiments, which resulted in 1 mM NaCl +5 mM KCl or 6 mM NaCl, respectively. Comparable amount of MilliQ water was added to cells as the negative controls (1 mM NaCl + MilliQ). The uptake rates were calculated by subtracting data of the empty pSE2 vector (25∼30 nmol mg total protein-1 hour-1) from raw data for each transporter. Values in the figure are means ± SD (n = 6).
(TIF)
Detection of SbtA7942 and SbtB7942 proteins in E. coli by western blotting. Gene expression was induced for 2.5 h with 1 mM IPTG. The soluble protein (S) and the membrane-enriched protein (M) fractions of E. coli containing the empty pSE2, 7942AB-HAH6 and 7942B-HAH6 vectors were used. A total of 30 µg total protein of each fraction per lane was separated by SDS-PAGE and subjected to Western blotting. Proteins were detected with the antibody cocktail of the SbtA antibody and the anti-HA antibody. * = SbtA monomer; # = SbtB monomer; ## = possible dimer of SbtB.
(TIF)
Detection of BicA7002 protein in the plasma membrane of E. coli by western blotting. Gene expression was induced for 2.5 h with 1 mM IPTG. The membrane-enriched protein fractions of E. coli containing the empty pSE2 and BicA7002 vectors were used. A total of 30 µg total protein of each fraction per lane was separated by SDS-PAGE and subjected to Western blotting. Proteins were detected with the antibody targeting the STAS domain of BicA. * = BicA monomer; ** = possible dimer of BicA.
(TIF)
Optimisation of the induction time required for expression of SbtA7942 and SbtA7001. Cultures were prepared as described in Materials and Methods. Expression of SbtA7942 (diamond) and SbtA7001 (triangle) was induced by adding IPTG (1 mM) for up to 5 hours with samples taken every hour to determine uptake rates. Uptake experiments were performed in the presence of 50 mM NaCl and 50 µM H14CO3 -. Net uptake was calculated by subtracting data of pSE2empty vector (25∼30 nmol mg total protein−1 hour−1) from raw data for each transporter. Values in the figure are means ± SD (n = 6).
(TIF)
Uptake time course for SbtA7942 and SbtA7001. Cultures were prepared as described in Materials and Methods. Uptake experiments were done in the presence of 50 mM NaCl and 50 µM H14CO3 -. Cells were incubated with H14CO3 - for 0.5, 1, 2 and 4 mins. Net uptake was calculated by subtracting data of pSE2empty control (0.38∼0.55 nmol mg total protein−1) from raw data of SbtA7942 (diamond) and SbtA7001 (triangle). Values in the figure are means ± SD (n = 6).
(TIF)
An alignment of the six SbtA forms used in the present study. The clones from β-cyanobacteria were Synechococcus elongatus sp. PCC7942 (SynPCC7942; freshwater), Synechococcus elongatus sp. PCC7002 (SynPCC7002; coastal/estuarine) and Synechocystis sp. PCC6803 (SycPCC6803; freshwater). The clones from α-cyanobacterial transitions strains were from Cyanobium spp. PCC6307 (CynPCC6307) and PCC7001 (CynPCC7001) and from Synechococcus WH5701 (SynWH5701). The positions of the membrane helices previously determined for Synechocystis PCC6803 SbtA are shown in purple. The conserved epitope region used for raising an antibody is shown in red. Residues are shaded according the functional categories: hydrophobic (green), positively charged (red), polar (orange) and aromatic (blue).
(TIF)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding provided by an Australian Research Council to GDP. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Herrera-Estrella LR (2000) Genetically Modified Crops and Developing Countries. Plant Physiol 124:923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tilman D, Balzer C, Hill J, Befort BL (2011) Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci U S A 108:20260–20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu X-G, Long SP, Ort DR (2010) Improving Photosynthetic Efficiency for Greater Yield. Ann Rev Plant Biol 61:235–261. [DOI] [PubMed] [Google Scholar]
- 4.Kimball BA, Kobayashi K, Bindi M (2002) Responses of Agricultural Crops to Free-Air CO2 Enrichment. In: Donald LS, editor. Advances in Agronomy: Academic Press. pp. 293–368.
- 5. Long SP, Zhu X-G, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29:315–330. [DOI] [PubMed] [Google Scholar]
- 6. Price GD, Badger MR, Woodger FJ, Long BM (2008) Advances in understanding the cyanobacterial CO2-concentrating-mechanism (CCM): functional components, Ci transporters, diversity, genetic regulation and prospects for engineering into plants. J Exp Bot 59:1441–1461. [DOI] [PubMed] [Google Scholar]
- 7. Price GD, Badger MR, von Caemmerer S (2011) The Prospect of Using Cyanobacterial Bicarbonate Transporters to Improve Leaf Photosynthesis in C3 Crop Plants. Plant Physiol 155:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Price GD, Pengelly JJL, Forster B, Du J, Whitney SM, von Caemmerer S, et al. (2013) The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J Exp Bot 64:753–768. [DOI] [PubMed] [Google Scholar]
- 9. Price GD, Shelden MC, Howitt SM (2011) Membrane topology of the cyanobacterial bicarbonate transporter, SbtA, and identification of potential regulatory loops. Molec Memb Biol 28:265–275. [DOI] [PubMed] [Google Scholar]
- 10. Rae BD, Long BM, Whitehead LF, Förster B, Badger MR, et al. (2013) Cyanobacterial carboxysomes: microcompartments that facilitate CO2 fixation. J Molec Microbiol Biotech 23:300–307. [DOI] [PubMed] [Google Scholar]
- 11. Price GD, Woodger FJ, Badger MR, Howitt SM, Tucker L (2004) Identification of a SulP-type bicarbonate transporter in marine cyanobacteria. Proc Natl Acad Sci U S A 101:18228–18233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shibata M, Katoh H, Sonoda M, Ohkawa H, Shimoyama M, et al. (2002) Genes essential to sodium-dependent bicarbonate transport in cyanobacteria - Function and phylogenetic analysis. J Biol Chem 277:18658–18664. [DOI] [PubMed] [Google Scholar]
- 13. Rae BD, Förster B, Badger MR, Price GD (2011) The CO2-concentrating mechanism of Synechococcus WH5701 is composed of native and horizontally-acquired components. Photosyn Research 109:59–72. [DOI] [PubMed] [Google Scholar]
- 14. Schwarz D, Nodop A, Hüge J, Purfürst S, Forchhammer K, et al. (2011) Metabolic and transcriptomic phenotyping of inorganic carbon acclimation in the cyanobacterium Synechococcus elongatus PCC7942. Plant Physiol 155:1640–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang P, Battchikova N, Jansen T, Appel J, Ogawa T, et al. (2004) Expression and functional roles of the two distinct NDH-1 complexes and the carbon acquisition complex NdhD3/NdhF3/CupA/Sll1735 in Synechocystis sp PCC 6803. Plant Cell 16:3326–3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Price GD, Howitt SM (2014) Topology mapping to characterize cyanobacterial bicarbonate transporters: BicA (SulP/SLC26 family) and SbtA. Molec Memb Biol 31:177–82. [DOI] [PubMed] [Google Scholar]
- 17. Pengelly JJL, Förster B, von Caemmerer S, Badger MR, Price GD, et al. (2014) Transplastomic integration of a cyanobacterial bicarbonate transporter into tobacco chloroplasts. J Exp Bot 65:3071–3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Badger MR, Hanson D, Price GD (2002) Evolution and diversity of CO2 concentrating mechanisms in cyanobacteria. Funct Plant Biol 29:161–173. [DOI] [PubMed] [Google Scholar]
- 19. Whitehead L, Long BM, Price GD, Badger MR (2014) Comparing the in vivo function of α-carboxysomes and β-carboxysomes in two model cyanobacteria. Plant Physiol 165:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Omata T, Price GD, Badger MR, Okamura M, Gohta S, et al. (1999) Identification of an ATP-binding cassette transporter involved in bicarbonate uptake in the cyanobacterium Synechococcus sp strain PCC7942. Proc Natl Acad Sci U S A 96:13571–13576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Merlin C, Masters M, McAteer S, Coulson A (2003) Why is carbonic anhydrase essential to Escherichia coli? J Bacteriol 185:6415–6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shelden MC, Howitt SM, Price GD (2010) Membrane topology of the cyanobacterial bicarbonate transporter, BicA, a member of the SulP (SLC26A) family. Molec Memb Biol 27:12–22. [DOI] [PubMed] [Google Scholar]
- 23.Imamura T (2006) Encyclopedia of Surface and Colloid Science. New York, Taylor & Francis.
- 24. Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM (2009) Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci U S A 106:1760–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Radchenko M, Merrick M (2011) The role of effector molecules in signal transduction by PII proteins. Biochem Soc Trans 39:189–194. [DOI] [PubMed] [Google Scholar]
- 26. Conroy MJ, Durand A, Lupo D, Li XD, Bullough PA, et al. (2007) The crystal structure of the Escherichia coli AmtB-GlnK complex reveals how GlnK regulates the ammonia channel. Proc Natl Acad Sci U S A 104:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Durand A, Merrick M (2006) In vitro analysis of the Escherichia coli AmtB-GlnK complex reveals a stoichiometric interaction and sensitivity to ATP and 2-oxoglutarate. J Biol Chem 281:29558–29567. [DOI] [PubMed] [Google Scholar]
- 28. Evans JR, von Caemmerer S (1996) Carbon dioxide diffusion inside Leaves. Plant Physiol 110:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karley AJ, Leigh RA, Sanders D (2000) Where do all the ions go? The cellular basis of differential ion accumulation in leaf cells. Trends Plant Sci 5:465–470. [DOI] [PubMed] [Google Scholar]
- 30. Rolland N, Ferro M, Seigneurin-Berny D, Garin J, Douce R, et al. (2003) Proteomics of chloroplast envelope membranes. Photosyn Res 78:205–230. [DOI] [PubMed] [Google Scholar]
- 31. Maeda S-I, Kawaguchi Y, Ohe T-A, Omata T (1998) cis-acting sequences required for NtcB-dependent, nitrite-responsive positive regulation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. Strain PCC 7942. J Bacteriol 180:4080–4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Joyce AR, Reed JL, White A, Edwards R, Osterman A, et al. (2006) Experimental and computational assessment of conditionally essential genes in Escherichia coli . J Bacteriol 188:8259–8271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Price GD, Badger MR (1989) Ethoxyzolamide inhibition of CO2 uptake in the cyanobacterium Synechococcus PCC7942 without apparent inhibition of internal carbonic anhydrase activity. Plant Physiol 89:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maeda S, Price GD, Badger MR, Enomoto C, Omata T (2000) Bicarbonate binding activity of the CmpA protein of the cyanobacterium Synechococcus sp strain PCC 7942 involved in active transport of bicarbonate. J Biol Chem 275:20551–20555. [DOI] [PubMed] [Google Scholar]
- 35. Yokota A, Kitaoka S (1985) Correct pK values for dissociation constant of carbonic acid lower the reported Km values of ribulose bisphosphate carboxylase to half. Presentation of a nomograph and an equation for determining the pK values. Biochem Biophys Research Comms 131:1075–1079. [DOI] [PubMed] [Google Scholar]
- 36. Zwaig N, Kistler WS, Lin ECC (1970) Glycerol Kinase, the pacemaker for the dissimilation of glycerol in Escherichia coli . J Bacteriol 102:753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Narayanan M, Gabrieli DJ, Leung SA, Elguindy MM, Glaser CA, et al. (2013) Semiquinone and cluster N6 Signals in His-tagged proton-translocating NADH:Ubiquinone oxidoreductase (Complex I) from Escherichia coli . J Biol Chem 288:14310–14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The effect of KCl and NaCl on the HCO3- uptake rates by SbtAs. Cells were prepared as described in the Methods except that a modified CO2-free buffer with 1 mM NaCl was used. Five mM KCl or NaCl was added to cells before the uptake experiments, which resulted in 1 mM NaCl +5 mM KCl or 6 mM NaCl, respectively. Comparable amount of MilliQ water was added to cells as the negative controls (1 mM NaCl + MilliQ). The uptake rates were calculated by subtracting data of the empty pSE2 vector (25∼30 nmol mg total protein-1 hour-1) from raw data for each transporter. Values in the figure are means ± SD (n = 6).
(TIF)
Detection of SbtA7942 and SbtB7942 proteins in E. coli by western blotting. Gene expression was induced for 2.5 h with 1 mM IPTG. The soluble protein (S) and the membrane-enriched protein (M) fractions of E. coli containing the empty pSE2, 7942AB-HAH6 and 7942B-HAH6 vectors were used. A total of 30 µg total protein of each fraction per lane was separated by SDS-PAGE and subjected to Western blotting. Proteins were detected with the antibody cocktail of the SbtA antibody and the anti-HA antibody. * = SbtA monomer; # = SbtB monomer; ## = possible dimer of SbtB.
(TIF)
Detection of BicA7002 protein in the plasma membrane of E. coli by western blotting. Gene expression was induced for 2.5 h with 1 mM IPTG. The membrane-enriched protein fractions of E. coli containing the empty pSE2 and BicA7002 vectors were used. A total of 30 µg total protein of each fraction per lane was separated by SDS-PAGE and subjected to Western blotting. Proteins were detected with the antibody targeting the STAS domain of BicA. * = BicA monomer; ** = possible dimer of BicA.
(TIF)
Optimisation of the induction time required for expression of SbtA7942 and SbtA7001. Cultures were prepared as described in Materials and Methods. Expression of SbtA7942 (diamond) and SbtA7001 (triangle) was induced by adding IPTG (1 mM) for up to 5 hours with samples taken every hour to determine uptake rates. Uptake experiments were performed in the presence of 50 mM NaCl and 50 µM H14CO3 -. Net uptake was calculated by subtracting data of pSE2empty vector (25∼30 nmol mg total protein−1 hour−1) from raw data for each transporter. Values in the figure are means ± SD (n = 6).
(TIF)
Uptake time course for SbtA7942 and SbtA7001. Cultures were prepared as described in Materials and Methods. Uptake experiments were done in the presence of 50 mM NaCl and 50 µM H14CO3 -. Cells were incubated with H14CO3 - for 0.5, 1, 2 and 4 mins. Net uptake was calculated by subtracting data of pSE2empty control (0.38∼0.55 nmol mg total protein−1) from raw data of SbtA7942 (diamond) and SbtA7001 (triangle). Values in the figure are means ± SD (n = 6).
(TIF)
An alignment of the six SbtA forms used in the present study. The clones from β-cyanobacteria were Synechococcus elongatus sp. PCC7942 (SynPCC7942; freshwater), Synechococcus elongatus sp. PCC7002 (SynPCC7002; coastal/estuarine) and Synechocystis sp. PCC6803 (SycPCC6803; freshwater). The clones from α-cyanobacterial transitions strains were from Cyanobium spp. PCC6307 (CynPCC6307) and PCC7001 (CynPCC7001) and from Synechococcus WH5701 (SynWH5701). The positions of the membrane helices previously determined for Synechocystis PCC6803 SbtA are shown in purple. The conserved epitope region used for raising an antibody is shown in red. Residues are shaded according the functional categories: hydrophobic (green), positively charged (red), polar (orange) and aromatic (blue).
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.


