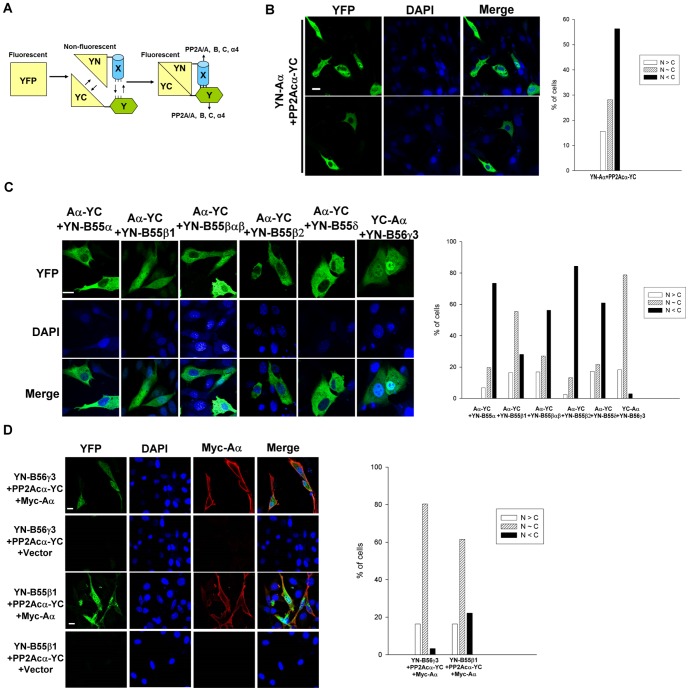
Figure 2. BiFC analysis enables visualization of association between two subunits of PP2A in cells.
(A) Design of BiFC analysis of dimeric interactions between PP2A subunits is shown. Fluorescence is regained when reconstitution of YFP from two fragments of YFP takes place due to an interaction between PP2A subunits fused to the fragments. (B) Equal amounts of BiFC expression constructs encoding YN-Aα and PP2Acα-YC were co-transfected into NIH3T3 cells. YFP signals due to BiFC of YN-Aα and PP2Acα-YC were measured by fluorescence microscopy. (C) Equal amounts of BiFC expression constructs encoding Aα-YC and YN-B55α, YN-B55β1, YN-B55β2, YN-B55βαβ, or YN-B55δ, or constructs encoding YC-Aα and YN-B56γ3 were transfected into NIH3T3 cells. YFP signals due to BiFC of Aα-YC and YN-B were measured by fluorescence microscopy. (D) Equal amounts of BiFC expression constructs encoding PP2Acα-YC and YN-B55β1 or YN-B56γ3 with or without equal amounts of pCA2-6myc-PP2A/Aα were co-transfected into NIH3T3 cells, and 24 h after transfection, YFP signals due to BiFC of PP2Acα-YC and YN-B55β1 or YN-B56γ3 were measured by direct fluorescence microscopy and expression of 6myc-PP2A/Aα was confirmed by indirect immunofluorescence using anti-Myc tag antibody and Cy3-conjugated secondary antibody. DAPI was applied for staining of nuclei. Scale bars: 20 µm. Graphs show quantitative analysis of distribution of BiFC signals in cells from one of at least two independent experiments with similar results, and at least 100 cells were assessed from several random fields. Cells with different distribution patterns of BiFC signals were scored as described earlier.