Figure 2.
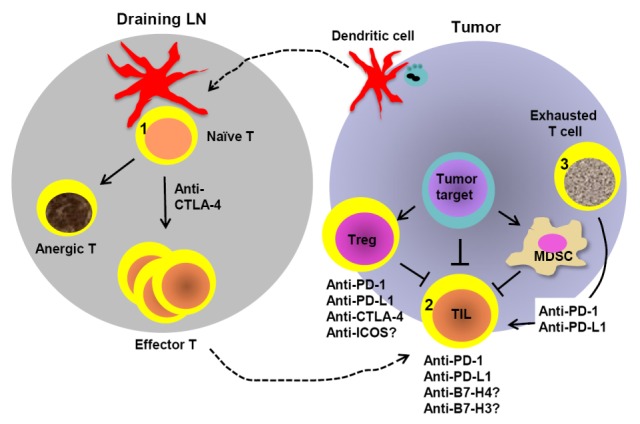
Stages of the anti-tumor T cell response in which B7 and CD28 blockade may function. In the initial T cell priming phase, DCs migrate from the tumor microenvironment to draining lymph nodes and present tumor antigens to naïve T cells. During this process, co-stimulatory signals provided by B7-1 and B7-2 to CD28 receptors prevent T cell anergy. (1) CTLA-4 plays a significant role in diminishing CD28-mediated co-stimulation, and inhibition of CTLA-4 has been shown to enhance the expansion of T cells specific to tumor-associated antigens. (2) Following activation, effector T cells infiltrate into the tumor to exert their functions, yet a host of immunosuppressive factors (such as MDSCs and Tregs) can dampen the anti-tumor response. Tumor cells and Tregs routinely upregulate co-inhibitory molecules such as PD-L1, B7-H3, B7-H4 and CTLA-4, weakening T cell immunity. Abrogation of these pathways results in enhanced anti-tumor responses, whereas the engagement of ICOS on TILs may be required for the persistence of tumor-specific T cells. (3) Lastly, elevated PD-L1 on tumor and/or infiltrating immune cells leads to T cell exhaustion, which can be reversed by PD-1 or PD-L1 blockade. Combinatorial immunotherapies disrupting multiple immunosuppressive mechanisms are predicted to improve anti-tumor efficacy.
