Abstract
Objective
To improve the efficiency and appropriateness of CT use in children with minor head trauma, clinical prediction rules were derived and validated by the Pediatric Emergency Care Applied Research Network (PECARN). The objective of this study was to conduct a cost-effectiveness analysis comparing the PECARN traumatic brain injury (TBI) prediction rules to usual care for selective CT use.
Methods
We used decision analytic modelling to project the outcomes, costs, and the cost-effectiveness of applying the PECARN rules compared with usual care in a hypothetical cohort of 1,000 children with minor blunt head trauma. Clinical management was directed by level of risk as specified by the presence or absence of variables in the PECARN TBI prediction rules. Immediate costs of care (diagnostic testing, treatment [not including clinician time], and hospital stay) were derived on single center data. Quality-adjusted life year (QALY) losses related to the sequelae of clinically important TBI (ciTBI) and to radiation-induced cancers, number of CT scans, radiation-induced cancers, missed ciTBI, and total costs were evaluated.
Results
Compared to the usual care strategy, the PECARN strategy was projected to miss slightly more children with ciTBIs (0.26 vs. 0.02 per 1000 children), but used fewer cranial CT scans (274 vs. 353), resulted in fewer radiation-induced cancers (0.34 vs. 0.45), cost less ($904,940 vs. $954,420), and had lower net QALY loss (−4.64 vs. −5.79). Because the PECARN strategy was more effective (less QALY loss) and less costly, it dominated the usual care strategy. Results were robust under sensitivity analyses.
Conclusion
Application of the PECARN TBI prediction rules for children with minor head trauma would lead to beneficial outcomes and more cost-effective care.
Background
The use of computed tomography (CT) in children has doubled over the last two decades, from 10.6 CTs per 1000 children in 1996 to 21.5 CTs per 1000 children in 2010.1 (Miglioretti DL, personal communication) Ionizing radiation is particularly worrisome in children; it is estimated that 1 in 1000 to 1 in 5000 cranial CT scans result in a later lethal cancer, with highest risks for younger children.2–4 To improve the efficiency and appropriateness of CT use in children with minor head trauma, clinical prediction rules were derived and validated by the Pediatric Emergency Care Applied Research Network (PECARN) to help clinicians with CT decision-making.5 The PECARN traumatic brain injury (TBI) prediction rules (one for children younger than 2 years, and the other for those 2 years and older) categorize the risk of clinically-important TBI (ciTBI) as high, intermediate, and low based on six clinical characteristics; ciTBI is defined as TBI on CT leading to intubation for more than 24 hours, hospital admission of 2 nights or more in association with a positive CT, need for neurosurgery, or death from TBI. If children in the low-risk category in the PECARN rules were to forego CT, without any other changes in practice, it is estimated that pediatric CT use for minor head trauma would decrease by 20–25% while rarely missing a child with ciTBI.5
Importance
The tradeoff between long term adverse effects of CT, the potential consequences of missed ciTBI, and the potential impact on health care costs has not been formally evaluated. Given the very long time horizon required to evaluate the potential consequences of radiation-induced cancers, we used decision modeling to compare the outcomes and costs of usual care to the outcomes and costs of application of the PECARN rules for the emergency care of children presenting with minor head trauma. Within the realm of cost-effectiveness analyses in health care, decision analytic models are a complementary tool to assess the relative efficiency of alternative management strategies under conditions of uncertainty. They are a necessary and valid component of assessing the tradeoffs between costs and benefits of different strategies as they bring costs, outcomes, probabilities and assumptions from multiple sources together.
Goals of This Investigation
We hypothesized that compared to usual care, implementation of the PECARN rules would result in overall higher quality of life and would be a cost-effective strategy.
METHODS
Study Design
We used decision analytic modelling to project the outcomes, costs, and the cost-effectiveness of applying the PECARN TBI prediction rules for selective CT use compared with usual care in a hypothetical cohort of 1,000 children (younger than 18 years old) with minor blunt head trauma (defined as a Glasgow Coma Scale (GCS) scores of 14–15 at emergency department [ED] presentation). The characteristics of this cohort were based on the PECARN TBI public use dataset.5 We included all children younger than 18 years, by combining the two PECARN age-specific rules, as they each consist of 6 variables and are applied in a similar fashion. We used a societal perspective over a lifetime horizon from which all outcomes were projected. Mean life expectancy was derived from US Vital Statistics life tables.6
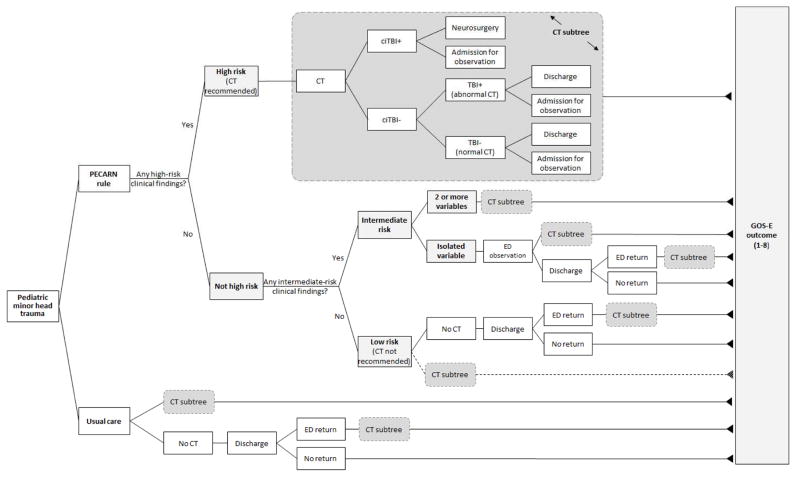
A simplified schematic of the decision model is presented in Figure 1. In the base case analysis of the PECARN strategy, clinical management was directed by level of risk as specified by the presence or absence of variables in the PECARN TBI prediction rules (eTable 1). High-risk children (who had a 4.4% risk of ciTBI in the PECARN study) were modelled to all receive an immediate ED cranial CT scan while all low-risk children (0.04% risk of ciTBI in the PECARN study) were modelled to not receive a CT scan and were discharged home from the ED. Intermediate-risk children (0.9% risk of ciTBI in the PECARN study) were modelled to receive either an immediate ED CT scan (in the presence of 2 or more PECARN intermediate risk variables, 2.1% risk of ciTBI) or ED observation before further decision-making (in the presence of a single PECARN intermediate risk variable, 0.6% risk of ciTBI). The management of children with minor head trauma in the PECARN data (by clinicians not applying the PECARN rules) defined the usual care strategy. Children who were modelled to receive a CT scan were categorized into outcomes of ciTBI, TBI on CT (but not clinically important), or normal based on previously published definitions (eTable 2).5 For both strategies, children who did not receive an initial ED CT scan but who were subsequently diagnosed with a ciTBI were considered to have a missed ciTBI. The decision model was programmed in TreeAge Pro 2011 (TreeAge Software, Inc., Williamstown, MA).
Figure 1.
Model Schematic
Abbreviations: CT, computed tomography; TBI, traumatic brain injury; ciTBI, clinically important TBI; GOS-E, extended Glasgow outcome score (see eTables 4 and 5)
Gray rounded rectangles share the same structure with the module denoted ‘CT subtree’.
Model Assumptions
We assumed cranial CT imaging was 100% sensitive and specific to detect TBI. Cranial CT is considered the criterion standard to detect TBI in children with minor head trauma and it is rare (less than 0.1%) for children with a normal cranial CT to be subsequently diagnosed with TBI.7 Prior cost-effectiveness studies have taken a similar approach of assuming that CT scanning has perfect test characteristics in identifying intracranial lesions requiring neurosurgical interventions.8,9 We assumed patients without an initial cranial CT in the ED are discharged home. This assumption is consistent with standard management of children with minor head trauma. In the PECARN dataset, in children with isolated head trauma, less than 0.2% of hospitalized children did not receive an initial cranial CT in the ED. We also assumed all patients with an abnormal CT scan (ciTBI or TBI) were admitted to the hospital. In the PECARN dataset, over 90% of children with abnormal CT scans were admitted to the hospital.
Probabilities
We derived probabilities conditional on children’s true disease status (ciTBI, TBI on CT, or normal) from PECARN study data (combined derivation and validation cohorts in both age groups; data collected from June 2004 to September 2006).5 Probabilities for the usual care cohort represent proportions observed in the PECARN study. For the PECARN prediction rules cohort, we derived the probabilities for each branch after applying the clinical prediction rules to the PECARN study population in a manner depicted in Figure 1, with the results shown in Table 1. We based the probability of future radiation-induced cancer caused by a single cranial CT scan on age-stratum-specific estimates of study population (eTable 3).2,4,10
Table 1.
| Parameter Name | Value
|
||||
|---|---|---|---|---|---|
| Prevalence of ciTBI | 0.89 (0.80–0.99) | ||||
| Prevalence of TBI | 0.96 (0.87–1.06) | ||||
| Lifetime risk of radiation induced malignancy due to single head CT exposure c | 0.13 (0.07–0.20) | ||||
| Chance of: | Usual Care |
PECARN Rules
|
|||
| being in the specified risk group |
High Risk
|
Intermediate Risk
|
Low Risk
|
||
| if patient has ciTBI | N/A | 68.7 (63.5–73.2) | 28.4 (24.1–33.1) | 2.9 d | |
| if patient has TBI | N/A | 48.3 (43.5–53.3) | 41.6 (36.9–46.5) | 10.5 d | |
| if patient has no ciTBI or TBI | N/A | 13.2 (12.8–13.5) | 29.6 (29.2–30.0) | 58.4 d | |
| being in the specified subgroup |
≥2 variables
|
isolated variable
|
|||
| if patient has ciTBI | N/A | N/A | 41.1 (32.3–50.6) | 58.9 d | N/A |
| if patient has TBI | N/A | N/A | 38.6 (31.5–46.0) | 61.4 d | N/A |
| if patient has no ciTBI or TBI | N/A | N/A | 16.0 (15.3–16.6) | 84.0 d | N/A |
| receiving an initial head CT in the ED | |||||
| if patient has ciTBI | 99.7 (98.5–100.0) | 100.0 | 100.0 | 100.0 | N/A |
| if patient has TBI | 99.0 (97.5–99.6) | 100.0 | 100.0 | 96.2 (90.5–98.5) | N/A |
| if patient has no ciTBI or TBI | 33.8 (33.4–34.3) | 100.0 | 100.0 | 31.5 (30.6–32.4) | N/A |
| hospital admission (otherwise discharged) after initial CT | |||||
| if patient has ciTBI | 100.0 | 100.0 | 100.0 | 100.0 | N/A |
| if patient has TBI | 79.7 (75.5–83.3) | 84.3 (78.6–88.7) | 78.5 (67.0–86.7) | 79.8 (71.1–86.4) | N/A |
| if patient has no ciTBI or TBI | 17.7 (17.0–18.3) | 25.8 (24.5–27.1) | 22.4 (20.6–24.3) | 0.0 | N/A |
| neurosurgery for ciTBI | 15.9 (12.6–20.0) | 15.1 (11.2–19.9) | 6.8 (2.4–18.2) | 27.0 (17.6–39.9) | N/A |
| returning to hospital for ongoing issue | |||||
| if patient has ciTBI | 100.0 | N/A | N/A | 100.0 | 100.0 |
| if patient has TBI | 100.0 | N/A | N/A | 100.0 | 100.0 |
| if patient has no ciTBI or TBI | 0.34 (0.28–0.42) | N/A | N/A | 0.63 (0.47–0.84) | 0.05 (0.03–0.09) |
| hospital admission after returning to hospital | |||||
| if patient has ciTBI | 100.0 | N/A | N/A | 100.0 | 100.0 |
| if patient has TBI | 100.0 | N/A | N/A | 100.0 | 100.0 |
| if patient has no ciTBI or TBI | 0.0 | N/A | N/A | 0.0 | 0.0 |
Abbreviations: TBI, traumatic brain injury; ciTBI, clinically important traumatic brain injury; CI, confidence interval; CT, computed tomography
- parameters with 95% CI presented were assigned a beta distribution in probabilistic sensitivity analysis
- data derived from PECARN data set 5
- not assigned a distribution in probability sensitivity analysis, instead the probabilities add up to 100% with probabilities sampled for other groups
Outcomes
The primary outcome was expressed as quality-adjusted life year (QALY) losses related to the sequelae of ciTBI and to radiation-induced cancers.11 Additional outcomes included: number of CT scans, radiation-induced cancers, missed ciTBIs, and total costs. Mean QALY losses from head injury were derived from probabilities and utility weights mapped to a pediatric global functional performance after TBI scale (six-month Glasgow Outcome Scale-extended Pediatrics [GOS-E Peds]) and estimated for five groups of patients (ciTBI [with and without neurosurgery], TBI on CT, normal CT, and missed ciTBI) within the model. Utility weights were based on prior literature while probabilities of functional outcomes were derived from an institutional TBI database (eTable 4 and 5).12,13
Lifetime radiation-induced cancer QALY loss, derived from age-stratum-specific estimates and discounted at 3% per year, was 10.42 QALYs per cancer in the base case (eTable 3).2,4,10
Costs
Immediate costs of care (diagnostic testing, treatment, and hospital stay) were derived from the financial department at the study site and represent estimated institutional resource use. Costs for clinician time were not included. We assigned costs to individual units of resources used (eTable 6). Costs of future radiation-induced cancers from a single current-generation cranial CT scan were based on work by Kutikova et al.14 All costs were discounted at 3%; for radiation-induced cancers, the latency period was estimated at 30 years.10 We calculated the total costs for each of the six primary branches (categorized by CT use, hospitalization, and disease status) in the decision tree by summing the estimated resource use (Table 2). Indirect costs such as time lost from work were not included.
Table 2.
| End node | Cost ($) |
|---|---|
| No CT, no TBI or ciTBI, discharged home | 756 |
| CT, no TBI or ciTBI, discharged home | 950 |
| CT, no TBI or ciTBI, admitted | 2,024 |
| CT, TBI, admitted | 4,507 |
| CT, ciTBI, admitted, no neurosurgery | 4,638 |
| CT, ciTBI, neurosurgery | 8,061 |
Abbreviations: CT, computed tomography; TBI, traumatic brain injury; ciTBI, clinically important traumatic brain injury
- assigned a log-normal distribution in the probabilistic sensitivity analysis
- total costs differed slightly (range $4 to $14) for each of the end nodes of the PECARN strategy as the probability of ED observation differed between risk categories (see eTable 6 for cost and probability of ED observation)
– see eTable 6 and 7 for individual costs inputs and cost summaries
Net Benefit
We assessed the value of applying the PECARN strategy (compared to the usual care strategy) using an incremental net monetary benefit (NMB) framework. The NMB framework combines the incremental cost and benefit of a utility into a single measure. The benefit of a utility was converted into a dollar value using willingness to pay (WTP) per QALY. We used a commonly accepted WTP threshold of $50,000 per QALY in the base case analysis.15–17 In sensitivity analyses, however, we tested a range of WTP from $30,000 per QALY to $100,000 per QALY. The formula for the NMB calculation was as follows:
The NMB approach avoids ambiguity and difficulties that may arise in computing the incremental cost-effectiveness ratios, especially when negative incremental costs and/or negative incremental utility values are involved in the analyses. A positive NMB value indicates that the PECARN strategy is more cost-effective than the usual care strategy (i.e., the PECARN rules dominates usual care), whereas a negative NMB value indicates the opposite.
Sensitivity Analyses
To evaluate the uncertainty of the model projections, we conducted three different types of sensitivity analyses. First, model parameter uncertainty was addressed through probabilistic sensitivity analysis. We assigned beta distributions (best fit for binomial data) to all probability parameters, based on the point estimates and their 95% confidence intervals derived from the PECARN data. In each of the 3,000 iterations of the simulation we conducted, the value of an input parameter was sampled from a specified distribution. We assigned gamma distributions to decreased QALYs, with lower and upper bounds of 0 and 1, respectively, and log-normal distributions to the aggregated cost of each endpoint. Simulation findings were presented as a cost-effectiveness scatter plot.
Second, we used univariate (i.e., one way) sensitivity analyses to assess the generalizability of the model output. Univariate sensitivity analysis complements probabilistic sensitivity analysis by evaluating the effect on model projections of varying one input parameter at a time across a plausible range. For example, because the PECARN data were derived from pediatric hospitals staffed primarily by pediatric emergency medicine fellowship trained physicians, the assumed distributions for sensitivity and specificity of physician’s diagnostic accuracy may not be reflective of practices in other settings with different practitioners. We varied possible probability and cost parameters by up to 20% of their base case values, utility values to lower and upper limits of their 95% confidence intervals, duration of decreased quality-adjusted life years from 3 months to 2 years, and WTP from $30,000 to $100,000 per QALY.
Finally, we used threshold analysis to determine the values of key parameters at which the NMB became zero. A NMB of zero means that the PECARN rules are no better than the usual care strategy at the threshold of $50,000 per QALY. We evaluated clinical parameters (e.g., lifetime risk of radiation-induced malignancy) as well as potential practice deviations. For example, in the base case model, we assumed that children with no PECARN TBI prediction rules criteria present (i.e., very low risk patients) would not receive a CT scan. In real practice, however, a child may receive a CT scan even if his/her risk of ciTBI is identified by the clinical prediction rules as very low. To evaluate clinical practice variation, a proportion of very low risk children were assigned to receive cranial CT scans (the dotted line in Figure 1).
RESULTS
Base Case Analysis
The results of the base case analysis for a cohort of 1,000 children with minor head trauma are shown in Table 3. Compared to the usual care strategy, the PECARN strategy was projected to miss more children with ciTBIs (0.26 vs. 0.02 per 1000 children [approximately one child per 4000 children with minor head trauma]), but used fewer cranial CT scans (274 vs. 353), resulted in fewer radiation-induced cancers (0.34 to 0.45 [approximiately one child per 9000 children with minor head trauma]) cost less ($904,940 vs. $954,420), and had lower net QALY loss (−4.64 vs. −5.79). Because the PECARN strategy was more effective (less QALY loss) and less costly, it dominated the usual care strategy.
Table 3. Base Case Output.
(per 1,000 children with minor head trauma)
| PECARN Rules | Usual Care | Difference a | |
|---|---|---|---|
| CT scans, n b | 274 | 353 | 79 |
| Radiation-induced malignancy | 0.34 | 0.45 | 0.11 |
| Missed ciTBI (delayed treatment), n | 0.26 | 0.02 | −0.24 |
| Total cost | $904,940 | $954,420 | $49,480 |
| Total QALY loss | −4.64 | −5.79 | −1.15 |
| QALY loss due to missed ciTBI | −0.92 | −0.99 | −0.07 |
| QALY loss due to radiation-induced malignancy | −3.72 | −4.80 | −1.08 |
| Incremental cost-effectiveness ratio of usual care, as compared with PECARN rules c | −$43,030 (dominated) | ||
| Net monetary benefit of PECARN rules d | $106,980 |
Abbreviations: CT, computed tomography; ciTBI, clinically important traumatic brain injury; QALY, quality-adjusted life year; WTP, willingness-to-pay
- difference = usual care – PECARN rules
- including CT scans for those who return to hospital for ongoing issues
- incremental cost-effectiveness ratio = Δtotal cost / ΔQALY loss
- net monetary benefit = (Δtotal QALY * WTP per QALY) - Δtotal cost. WTP used here is $50,000/QALY.
Sensitivity Analysis
Probabilistic sensitivity analysis confirmed that the base case results were robust to variation in input parameters. In all 3,000 simulation iterations in the probabilistic sensitivity analysis, the PECARN strategy was more effective (less QALY loss) and less expensive (and thus dominant) than the usual care strategy (eFigure 1).
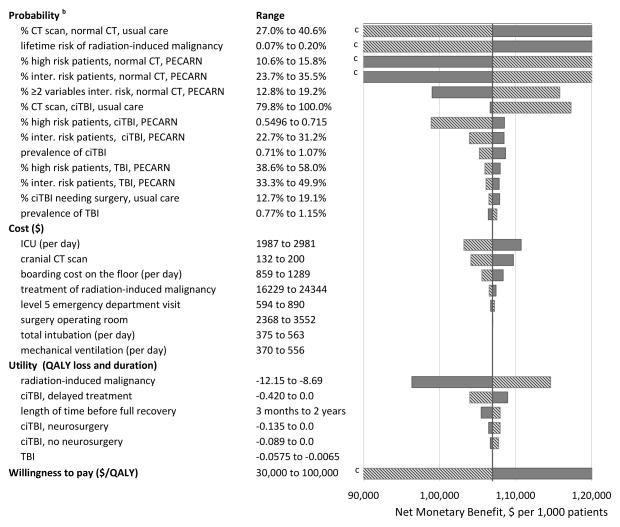
A tornado diagram (bar chart in which data are ordered vertically with the largest bar indicated the largest effect appearing at the top of the chart, the second largest appearing second from the top, and so on) stratified by parameter type is shown in Figure 2. Among the probability parameters, the NMB was most sensitive to change in probabilities of patients without TBI receiving immediate CT scans in the usual care strategy, followed by the lifetime risk of radiation-induced cancer. The NMB was also very sensitive to changes in the likelihood of normal patients being categorized into risk groups that suggested the need of CT (high risk group, and subgroup of 2 or more variables within the intermediate risk). The most influential cost inputs were ICU stay, cranial CT scan, and boarding cost on the floor. The most sensitive utility parameter was the QALY loss for radiation-induced cancer. The change of WTP to $30,000 per QALY would reduce the NMB by approximately 20%, whereas increasing WTP to $100,000 per QALY more than doubled the NMB. Finally, results of the threshold analysis demonstrated that key clinical parameters and variation in practice would have to be substantially changed to alter the results of the base case analysis (Table 4). For example, in the usual care strategy in the base case scenario, 33.8% of children with minor head trauma and no ciTBI or TBI received a cranial CT scan. The PECARN strategy is more cost-effective than the usual care strategy until the CT scan rate in the usual care strategy decreases to 26.0%.
Figure 2.
One-way sensitivity analysis: Tornado diagram a
Abbreviations: CT, computed tomography, ICU, intensive care unit; TBI, traumatic brain injury; ciTBI, clinically important traumatic brain injury; QALY, quality adjusted life year
a The solid and dashed lines represent the change of NMB when a parameter increases or decreases from its basecase value, respectively. The probability and cost parameters resulting in less than 3% and 1% change in net benefit, respectively, are not shown.
b - Parameter names with 3 parts separated by comma are conditional probabilities. The first part is the event, the second part is the injury status (including normal [i.e., not ciTBI or TBI], TBI, or ciTBI) which the event is conditional on, and the third part is the strategy (PECARN rules vs. usual care) in which the event is concerned. For example, ‘% CT scan, normal, usual care’ indicates the chance of receiving CT scan in the usual care strategy given the patient does not have TBI or ciTBI (i.e., normal).
c - Net monetary benefit (NMB) below $90,000 or above $120,000 is not shown. NMB for ‘% CT scan, normal, usual care’ varies from $34,154 to $180,333; NMB for ‘lifetime risk of radiation-induced malignancy’ varies from $77,359 to $141,254; NMB for ‘% high risk patients, normal, PECARN’ varies from $76,170 to $136,929; NMB for ‘% intermediate risk patients, normal, PECARN’ varies from $82,173 to $131,831; NMB for ‘willingness to pay’ varies from $83,984 to $221,984.
Table 4.
Threshold sensitivity analysis
| Parameter name | Base-case value | Threshold value a | Comment |
|---|---|---|---|
| % CT scan for normal patients in usual care strategy | 0.338 | 0.260 | In the usual care strategy of the base case scenario, 33.8% of children with minor head trauma and no ciTBI or TBI received a cranial CT scan. The PECARN strategy is more cost-effective than the usual care strategy until the usual care strategy CT scan rate decreases to 26.0%. |
| lifetime risk of radiation-induced malignancy | 0.132 | not existing | The PECARN strategy is cost effective even when not accounting for radiation-induced malignancy QALY loss. In this scenario, the PECARN strategy has more QALY loss from missed ciTBI but is still cost effective (i.e., net monetary benefit of PECARN strategy is greater than 0). |
| QALY loss for a missed ciTBI | 0.163 | 9.044 | The QALY loss for a missed ciTBI would need to be significantly greater than the base case scenario for the PECARN strategy to no longer be effective. This threshold value equates to 20% of missed ciTBI resulting in death b |
| Prevalence of ciTBI | 0.0089 | 0.2417 | When the prevalence of ciTBI is over 24.0% of children with minor head trauma the PECARN strategy is no longer cost-effective |
| % CT scan for low risk patients in PECARN strategy | 0 | 0.211 | The PECARN strategy is no longer cost-effective when clinicians obtain a CT scan for 21.1% of low risk patients in the PECARN strategy. |
Abbreviations: CT, computed tomography; TBI, traumatic brain injury; ciTBI, clinically important traumatic brain injury; ICER: incremental cost-effectiveness ratio; WTP: willingness to pay; QALY: quality adjusted life year; GOS-E: extended Glasgow outcome score.
- threshold value is where the cost-effective strategy switches from the PECARN rules strategy to the usual care strategy (i.e., net monetary benefit of PECARN rules equals 0).
- calculation is based on the assumption that children that die from a missed ciTBI lose all future QALYs. The amount of future QALYs is based on US life expectancy and weighted by age distribution in the study population, with a yearly 3% discount rate applied. QALY loss for death is 30.75.
LIMITATIONS
Our results should be interpreted in the context of certain limitations. The use of cost data from a single center might limit the generalizability to those in different settings (e.g., non-Level 1 trauma centers) or geographic locations, and clinician costs were not included. We explored the possibility of using national cost data from the Health Costs and Utilization Project, however, data limitations, including the lack of costs for non-hospitalized patients and the unreliability of reported CT imaging, prevented its use.18 Nevertheless, our results were robust with the same cost data being applied to both strategies as well as the use of sensitivity analyses with varying cost inputs (Figure 2).
Our model was designed to evaluate the implementation of the PECARN TBI prediction rules to all children with minor head trauma. We did not evaluate the cost-effectiveness across specific subgroups (such as patient age); it is therefore possible that the results may have varied between subgroups.
Real-world implementation of the PECARN TBI prediction rules strategy may produce different probabilities than those assigned in our model. For example, clinicians may obtain cranial CT scans in some patients who are very low risk by the PECARN rules. While we conducted a sensitivity analysis on this particular model assumption, we could not evaluate all potential differences in probabilities between our base case model and the possible variations in PECARN rule implementation in the real-world.
While the PECARN prediction rules were derived and independently validated in a diverse and large patient population (over 42,000 children with minor head trauma at more than 20 sites) allowing sufficient statistical power to generate robust and generalizable rules,19,20 it is possible that the rules may perform differently in other settings and by other practitioners, such as in non-academic centers and non-pediatric emergency medicine practitioners.21 However, our sensitivity analyses demonstrated that the accuracy of the PECARN rules and usual care strategies would have to be substantially lower to alter the results of the base case model. Furthermore, the clinical prediction rules were derived at children’s referral centers where clinicians are very experienced in the care of injured children (see eTable 8 for CT use by PECARN risk categories). The PECARN rules are likely to be more helpful to clinicians less experienced evaluating injured children.22,23
In addition to the PECARN rules, a recent systematic review identified two other high quality clinical prediction rules for the evaluation of children with minor head trauma: CATCH (Canadian Assessment of Tomography for Childhood Head Injury) and CHALICE (Children’s Head Injury Algorithm for the Prediction of Important Clinical Events).24–26 All were derived with high methodological standards but the PECARN rules are validated,24 and was recently cited as one of the 5 pediatric priorities in the “Choosing Wisely” campaign.27
DISCUSSION
Our cost-effectiveness analysis for the evaluation of children with minor head trauma projected that implementation of the PECARN prediction rules was associated with less frequent cranial CT use, fewer radiation-induced cancers, lower total costs, and lower total QALY loss compared to a strategy based on usual care. In our model, the PECARN strategy was dominant – it was more effective (less total QALY loss) and less costly than the usual care strategy. These results were robust under a number of sensitivity analyses.
Cost-effectiveness analyses using decision models should serve as tools for clinicians and policy makers to aid in decision making rather than as unconditional or conclusive results.28 Models are highly dependent on assumptions and the various inputs and should be continually assessed against new data.
Nevertheless, our findings highlight the importance of improving appropriate CT use, particularly for children. Clinical prediction rules may improve appropriate use and decrease costs; an estimated 1 million children are unnecessarily imaged with CT scans each year in the US.2 Children are at greater risk for radiation-induced cancers than adults from a given radiation dose because they are inherently more radiosensitive and they have more remaining years of life during which a radiation-induced cancer could develop.2 The risk of radiation-induced cancer for an infant receiving a single cranial CT scan is more than four times than that of an adult.10
From a public health perspective, unnecessary CT imaging leads to a substantial burden of disease, with associated costs to society. Based on estimates of the frequency of blunt head trauma in children, implementation of the PECARN TBI prediction rule strategy instead of usual care could prevent 60 radiation-induced cancers in children annually in the US.29 From an economic standpoint, implementation would reduce US hospital expenditures annually by approximately $27 million in the US.29
The potential downside of less frequent CT use by employing the PECARN strategy is the potential for clinicians to fail to identify some children with ciTBIs. This risk, however, is extremely small, projected as one additional missed ciTBI for every 4000 children evaluated for minor head trauma. This is consistent with the only other study we identified that described outcomes for children with missed TBIs.30 In that population-based study, the proportion of children with minor head trauma whose TBIs were initially missed was less than 1 per 100,000.30
In conclusion, compared to usual care, implementation of the PECARN TBI prediction rules in the evaluation of children with minor head trauma was projected to reduce total healthcare costs, lower cranial CT use, result in fewer radiation-induced cancers and lead to higher net QALYs and thus was the dominant strategy. These results were robust across a variety of sensitivity analyses and suggest that widespread application of the PECARN TBI prediction rules for children with minor head trauma would lead to beneficial outcomes and more cost-effective care.
Supplementary Material
Acknowledgments
DN was supported through a Mentored Clinical Research Training Program Award (KL2), Grant Number UL1 RR024146 from the National Center for Research Resources (now National Center for Advancing Translational Sciences [NCATS]), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The UC Davis School of Medicine, NCATS, and NIH had no role in the design and conduct of the study, in the analysis or interpretation of the data, or in the preparation of the data. The views expressed in this article are solely the responsibility of the authors and do not necessarily represent the official view of the NCATS, or the NIH. Information on NCATS is available at http://www.ncats.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
This manuscript was prepared using the “Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study” Data Set and does not necessarily reflect the opinions or views of the TBI Trial investigators, the Health Resources Services Administration (HRSA), the Maternal Child Health Bureau (MCHB), or Emergency Medical Services for Children (EMSC). The PECARN was funded by the HRSA/MCHB/EMSC.
Footnotes
Conflicts of Interest: None
Author Contributions:
All authors conceptualized and designed the study. DN, ZY, and MU acquired the data. DN, ZY, MU, JM, JH, and NK analyzed the data. DN drafted the manuscript and all authors contributed substantially to its revisions. DN takes responsibility for the paper as a whole.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatr. 2013;167(8):700–7. doi: 10.1001/jamapediatrics.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–84. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 3.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32:228–3. doi: 10.1007/s00247-002-0671-1. [DOI] [PubMed] [Google Scholar]
- 4.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380:499–505. doi: 10.1016/S0140-6736(12)60815-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuppermann N, Holmes JF, Dayan PS, et al. Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet. 2009;374:1160–70. doi: 10.1016/S0140-6736(09)61558-0. [DOI] [PubMed] [Google Scholar]
- 6.Arias E. National Vital Statistics Reports: United States Life Tables, 2007. Hyattsville, MD: National Center for Health Stasitics; 2011. [PubMed] [Google Scholar]
- 7.Holmes JF, Borgialli DA, Nadel FM, et al. Do children with blunt head trauma and normal cranial computed tomography scan results require hospitalization for neurologic observation? Ann Emerg Med. 2011;58:315–22. doi: 10.1016/j.annemergmed.2011.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Holmes MW, Goodacre S, Stevenson MD, et al. The cost-effectiveness of diagnostic management strategies for adults with minor head injury. Injury. 2012;43:1423–31. doi: 10.1016/j.injury.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Smits M, Dippel DW, Nederkoorn PJ, et al. Minor head injury: CT-based strategies for management--a cost-effectiveness analysis. Radiology. 2010;254:532–40. doi: 10.1148/radiol.2541081672. [DOI] [PubMed] [Google Scholar]
- 10.Stein SC, Hurst RW, Sonnad SS. Meta-analysis of cranial CT scans in children. A mathematical model to predict radiation-induced tumors. Pediatr Neurosurg. 2008;44:448–57. doi: 10.1159/000172967. [DOI] [PubMed] [Google Scholar]
- 11.Prieto L, Sacristan JA. Problems and solutions in calculating quality-adjusted life years (QALYs) Health Qual Life Outcomes. 2003;1:80. doi: 10.1186/1477-7525-1-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosty J, Macyszyn L, Lai K, et al. Relating quality of life to glasgow outcome scale health states. J Neurotrauma. 2012;29:1322–7. doi: 10.1089/neu.2011.2222. [DOI] [PubMed] [Google Scholar]
- 13.Beers SR, Wisniewski SR, Garcia-Filion P, et al. Validity of a pediatric version of the Glasgow Outcome Scale-Extended. J Neurotrauma. 2012;29:1126–39. doi: 10.1089/neu.2011.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kutikova L, Bowman L, Chang S, et al. Utilization and cost of health care services associated with primary malignant brain tumors in the United States. J Neurooncol. 2007;81:61–5. doi: 10.1007/s11060-006-9197-y. [DOI] [PubMed] [Google Scholar]
- 15.Bobinac A, van Exel NJ, Rutten FF, Brouwer WB. Valuing QALY gains by applying a societal perspective. Health Econ. 2013;22:1272–81. doi: 10.1002/hec.2879. [DOI] [PubMed] [Google Scholar]
- 16.Kirkdale R, Krell J, Brown CO, et al. The cost of a QALY. QJM. 2010;103:715–20. doi: 10.1093/qjmed/hcq081. [DOI] [PubMed] [Google Scholar]
- 17.Garber AM, Phelps CE. Economic foundations of cost-effectiveness analysis. J Health Econ. 1997;16:1–31. doi: 10.1016/s0167-6296(96)00506-1. [DOI] [PubMed] [Google Scholar]
- 18.Kids’ Inpatient Database (KID) Healthcare Costs and Utilization Project (HCUP) Rockville, MD: Agency for Healthcare Research and Quality; 2013. [Accessed June 10, 2013]. at http://www.hcup-us.ahrq.gov/kidoverview.jsp. [Google Scholar]
- 19.Maguire JL, Boutis K, Uleryk EM, et al. Should a head-injured child receive a head CT scan? A systematic review of clinical prediction rules. Pediatrics. 2009;124:e145–54. doi: 10.1542/peds.2009-0075. [DOI] [PubMed] [Google Scholar]
- 20.Stiell IG, Wells GA. Methodologic standards for the development of clinical decision rules in emergency medicine. Ann Emerg Med. 1999;33:437–47. doi: 10.1016/s0196-0644(99)70309-4. [DOI] [PubMed] [Google Scholar]
- 21.McGinn TG, Guyatt GH, Wyer PC, et al. Users’ guides to the medical literature: XXII: how to use articles about clinical decision rules. Evidence-Based Medicine Working Group. JAMA. 2000;284:79–84. doi: 10.1001/jama.284.1.79. [DOI] [PubMed] [Google Scholar]
- 22.Blackwell CD, Gorelick M, Holmes JF, et al. Pediatric head trauma: changes in use of computed tomography in emergency departments in the United States over time. Ann Emerg Med. 2007;49:320–4. doi: 10.1016/j.annemergmed.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Gausche-Hill M, Schmitz C, Lewis RJ. Pediatric preparedness of US emergency departments: a 2003 survey. Pediatrics. 2007;120:1229–37. doi: 10.1542/peds.2006-3780. [DOI] [PubMed] [Google Scholar]
- 24.Lyttle MD, Crowe L, Oakley E, et al. Comparing CATCH, CHALICE and PECARN clinical decision rules for paediatric head injuries. Emerg Med J. 2012;29:785–94. doi: 10.1136/emermed-2011-200225. [DOI] [PubMed] [Google Scholar]
- 25.Osmond MH, Klassen TP, Wells GA, et al. CATCH: a clinical decision rule for the use of computed tomography in children with minor head injury. CMAJ. 2010;182:341–8. doi: 10.1503/cmaj.091421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dunning J, Daly JP, Lomas JP, et al. Derivation of the children’s head injury algorithm for the prediction of important clinical events decision rule for head injury in children. Arch Dis Child. 2006;91:885–91. doi: 10.1136/adc.2005.083980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Five Things Physicians and Patients Should Question: The Choosing Wisely Initiative. American Academy of Pediatrics; 2013. [Accessed May 22, 2013]. at http://www.choosingwisely.org/doctor-patient-lists/american-academy-of-pediatrics/ [Google Scholar]
- 28.Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR Task Force on Good Research PracticesfModeling Studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 29.Faul MXL, Wal MM, Coronado VG. Traumatic brain injury in the United States: emergency department visits, hospitalizations, and deaths 2002–2006. US Department of Health and Human Services, CDC; Atlanta, GA: 2010. [Google Scholar]
- 30.Hamilton M, Mrazik M, Johnson DW. Incidence of delayed intracranial hemorrhage in children after uncomplicated minor head injuries. Pediatrics. 2010;126:e33–9. doi: 10.1542/peds.2009-0692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.