Abstract
Background:
There is abundance of literature delving into whether periodontal infection contributes to changes in serum lipid profiles. Whole saliva is an important physiologic fluid that contains a highly complex mixture of substances. Research on salivary lipid profiles and chronic periodontitis remains unexplored and limited. This study was designed with an aim to investigate the association between the chronic periodontitis and salivary lipid levels and to make use of saliva as a non-invasive diagnostic aid.
Materials and Methods:
This case-control study included 60 subjects of which, 40 were diagnosed as having chronic periodontitis based on the probing depth and clinical attachment levels and 20 healthy subjects as control group. Whole saliva was collected and lipid concentrations (total cholesterol (TC), triglycerides (TG), low density lipoprotein [LDL] and high density lipoprotein [HDL]) were assessed by enzymatic methods and the values were read in ultraviolet-Spectrophotometer. Data was analyzed using student's t test for equality of means. P < 0.05 was considered to be statistically significant.
Results:
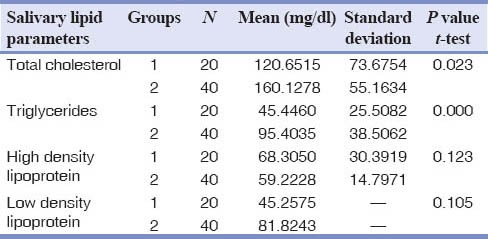
The mean difference in the concentrations of TC and TG in saliva of chronic periodontitis patients were statistically significant (P = 0.02) when compared to the healthy. HDL and LDL concentrations were not statistically significant, but there was a difference in their means. LDL was higher in chronic periodontitis and HDL mean levels were high among the healthy.
Conclusion:
Increased salivary lipids in chronic periodontitis patients suggest an association between hyperlipidemia and periodontitis. The relatively easy and non-invasive nature of saliva can be used as a diagnostic tool to assess the lipid status. Further research is needed to determine its specificity as a surrogate to serum lipid profiles.
Keywords: Chronic periodontitis, hyperlipidemia, lipids, saliva
INTRODUCTION
It is more than a century since a connection between the oral cavity and rest of the body first appeared in the medical literature. The notion of oral sepsis then termed as the “Focus of infection” was extensively debated among the dentist and physician. Numerous unknown etiologies were thought to be causally linked to the common oral infection such as, dental caries and pyorrhea. This theory fell into disrepute, when it was found that extraction failed to eliminate or reduce the systemic disease to which the infected teeth were linked.[1] This old concept is seeing new light now bridging the gap between the medicine and dentistry. Recent literature suggests that periodontal disease can produce disorders in systemic health by changing the blood chemistry with a rise in inflammatory mediators, proteins and lipids in the serum.[2]
Periodontitis is a persistent bacterial infection causing chronic inflammation in periodontal tissues. It is characterized by formation of pathological periodontal pockets concomitantly with destruction of periodontal ligament fibers attaching teeth to the alveolar bone and alveolar bone itself.[3]
Cardiovascular diseases are the main cause of death in the world, being responsible for 16% and 50% of deaths in developing and developed countries respectively.[4] Atherosclerosis is also very common and starts early in life, however, since disease progression is usually slow, clinical symptoms or hospitalization are rare before 40 years of age.[5] Since hyperlipidemia is a main risk factor for cardiovascular diseases, such as atherosclerosis, cardiac ischemic disorders, and strokes, it is imperative to determine its causes.[6] Its a state of abnormal lipid profile, which is characterized by elevated blood concentrations of triglycerides (TG), elevated levels of total cholesterol (TC) and low-density lipoprotein (LDL)-cholesterol and decreased levels of high-density lipoprotein (HDL)-cholesterol. HDL have several antiatherogenic properties, such as an ability to promote the efflux of cholesterol from cells, to function as an important antioxidant by inhibiting LDL oxidation, to prevent or interrupt foam cell formation and to retard inflammatory activity.[2]
Cardiovascular diseases and periodontitis have several risk factors in common and health awareness poses a particular challenge. Another possible mechanism accounting for the reported association between the periodontitis and cardiovascular disease could be the release of bacteria, bacterial products or pro-inflammatory cytokines (Interleukin 1 [IL-1], tumor necrosis factor-α [TNF-α]) from the chronic periodontal lesion into the blood stream. This might lead to a systemic inflammatory response, which resembles a risk factor profile that is consistent with cardiovascular disease.[1,5]
Saliva is considered as an ultra-filtrate of plasma. It can be easily collected with fewer compliance problems when compared with the collection of blood. The constituents of saliva are derived from the local vasculature of salivary glands.[7] Whole saliva is an important physiologic fluid that contains a highly complex mixture of substances. Variable amounts of blood, serum products are present in whole saliva.[8] The presence of lipids in saliva has been known since the work of Doubleday (1909).[9] The total lipid content of whole saliva was 2-3 μg/ml, which consisted of cholesterol, fatty acids and TG. Salivary lipids are mostly glandular in origin, but some believed to diffuse directly from serum. Karjalainen et al. assessed cholesterol in saliva of healthy adults; they concluded that salivary concentration levels reflect serum concentration to some extent.[10] Salivary lipids may have a nucleating role in the early mineralization of the dental plaque, i.e., in calculus formation. Thus, the accessibility of the mouth renders it as a portal through which one can potentially monitor systemic and oral health.[11] Whole saliva is most frequently studied when salivary analysis is used for the evaluation of systemic disorders.[12]
The prevalence of periodontal diseases may be increased in patients with atherosclerosis and have been shown to be associated with the occurrence of acute and chronic coronary heart disease.[10] Recent evidence suggests that common cardio-metabolic risk factors including body weight, dyslipidemia and hypertension as individual components or clustered in the metabolic syndrome are also associated with increased odds of prevalence of periodontitis.[4]
The aim of the present study was, first, to investigate the association between salivary levels of TC, TG, HDL and LDL and periodontitis and second, to make use of saliva as a non-invasive diagnostic aid.
MATERIALS AND METHODS
Subjects
The subjects for the study were selected from the Outpatient section, Department of Periodontics, PMNM Dental College and Hospital, Bagalkot. The Ethics Committee and Review Board of PMNM Dental College and Hospital, Bagalkot approved the study protocol. The protocol was clearly explained to the patients and informed consent was obtained from all the recruits.
This case-control study included all subjects with more than three pockets with a probing pocket depth (PPD) ≥ 4 mm or with a clinical attachment loss (CAL) ≥ 3 mm. The study population consisted of 60 subjects. These were divided into two groups: Healthy (control) group of 20 subjects (10 females and 10 males) and 40 subjects (17 females and 23 males) with chronic periodontitis with an age range of 30-65 years were enrolled in the study.
The patients who underwent dental treatment during the past 6 months, smokers, alcoholics, pregnant, lactating mothers and post-menopausal women, aggressive periodontitis patients, cardiac diseases, rheumatoid arthritis, obesity, patients taking any drugs against hypercholesterolemia and any other systemic disease which could alter the course of periodontal disease or lipid levels were excluded from the study.
Saliva sampling
Saliva samples were collected in the morning after the minimum of 12 h fasting over night before the clinical appointment in restful and quite circumstances, following flushing of mouth with 100 ml of tap water. The unstimulated whole saliva was collected for 5 min by asking the subject to lean forward and spit the saliva into epindroff tubes prior to clinical measurements. Immediately after collection, the samples were cold centrifuged at 3000 rpm at 4°C for 5 min. The supernatant was aspirated and stored at –20°C until analyzed.[7]
Laboratory analysis of salivary lipids
Salivary lipids were analyzed by enzymatic methods using the Erba diagnostic kits (Transasia Bio-Medicals, Solan, HP, Germany). TC concentration in saliva was analyzed by cholesterol oxidase-phenol aminophenazone method (Modified Roeschlau's method), TG concentrations measured using GPO-TRINDER method and HDL was measured by the Phosphotungstic acid method. LDL concentration was then calculated from the concentration of TC, HDL and TG using the method of Friedwald and Levy (LDL = TC-HDL-TG/5 [mg/dl]). After the biochemical analysis the values were read through ultraviolet-Spectrophotometer.
All data were expressed as means and standard deviations. The statistical difference in the mean between the groups was tested using Student's t-test. P < 0.05 was considered to be statistically significant.
RESULTS
The various salivary lipid parameters in the chronic periodontitis group and control group were analyzed. The TC and TG levels in saliva were substantially higher in the chronic periodontitis group when compared with the healthy group (P < 0.05). The mean concentration of LDL was high and the concentrations of HDL were low in chronic periodontitis group when compared to the healthy group. The TC, TG showed statistically significant results in chronic periodontitis group but HDL and LDL were no statistically significant yet showed a mean rise in LDL chronic periodontitis group. A fall in HDL levels in the chronic periodontitis group when compared to the healthy group was noticed [Table 1].
Table 1.
Salivary lipid levels in healthy and chronic periodontitis groups
DISCUSSION
A high serum lipid level is one of the modern society concerns and hyperlipidemia is considered as one of the major cardiovascular disease risk factors.[13] Chronic local and acute systemic infections have been shown to induce profound changes in the plasma concentration of cytokines leading to hyperlipidemia. This suggests that even oral infection, such as periodontitis has the potential to cause generalized alterations in lipid metabolism.[14]
The present study data indicates that periodontitis causes alteration in salivary TC, HDL, LDL and TG levels. This finding was in accordance with Lösche et al. which showed that mean TC and LDL levels of periodontitis patients were significantly higher by about 8% and 13% respectively when compared to controls.[15]
Chronic infections like periodontitis result in elevated levels of cytokines in response to Gram-negative microorganisms lipopolysaccharide exposure. Two principle cytokines involved in this process are, TNF-α and IL-1α. It is believed that these cytokines exert effects on lipid metabolism by production of other cytokines, altering the hemodynamics/amino acid utilization of various tissues involved in lipid metabolism or modifying concentration of various hormones. Thus, through the action of TNF-α and IL-1α, exposure to microorganisms or endotoxins results in elevated levels of serum lipids. This elevation in serum lipids is thought to arise from enhanced hepatic lipogenesis, increased adipose tissue lipolysis/blood flow, and increased synthesis or reduced clearance of LDL.[14]
Saliva is increasingly used and well-validated in diagnosing, monitoring systemic disease status and predicting disease progression. Biomarkers detected in saliva can be valuable in a wide range of clinical pathology, forensic medicine and sport medicine.[7] Low levels of salivary lipids are probably needed for the maintenance of good oral health, but excess may be harmful.[10]
The results obtained from the present study showed a statistical significant difference in salivary TC and TG levels (P < 0.05) among the healthy and chronic periodontitis groups. Even though, LDL and HDL levels did not show any statistical significant changes, there was a mean increase in LDL and decrease in HDL concentrations among chronic periodontitis group.
Lösche et al.[15] found only LDL was significantly associated with the clinical parameters of inflammation and periodontal tissue destruction in periodontitis subjects. Iacopino and Cutler[16] reported the existence of an association between periodontitis and serum lipid levels does not establish whether periodontal disease causes elevations in lipid levels or elevations in serum lipids predispose to periodontitis. Hence, these studies in actual fact do not depict a strong correlation between serum lipid levels and periodontal disease. A possible explanation for the association between hyperlipidemia and periodontal infection was postulated by Noack et al.[17] by assessing neutrophil respiratory burst by whole blood chemiluminescence. It was found that there was a significant increase in both chemiluminescence and pocket depth on a group of patients having hyperlipidemia and thus, suggested that the association of hyperlipidemia with periodontitis could be due to the dysfunction of polymorphonuclear leucocytes.
Within the limitations of this study, we found an association between salivary lipid profiles and chronic periodontitis, the etiology of hyperlipidemia is multifactorial and it is suggested to do true experimental design research to exactly confirm the effect of chronic periodontitis on hyperlipidemia. Our efforts would be continued toward focusing on the comparison between the serum and salivary lipids levels.
CONCLUSION
From the present study, it was concluded that there is a causal association between chronic periodontitis and salivary lipid profiles. Salivary diagnostics holds a tremendous promise for long-term goal of developing clinically validated saliva based tests for health surveillance and early detection of oral diseases and systemic conditions. Further studies are needed to determine the specificity of saliva as a surrogate for serum lipid profiles.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Gita B, Sajja C, Padmanabhan P. Are lipid profiles true surrogate biomarkers of coronary heart disease in periodontitis patients? A case-control study in a South Indian population. J Indian Soc Periodontol. 2012;16:32–6. doi: 10.4103/0972-124X.94601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doubleday AW. Plodding toward diagnosis by salivary analysis. Dent Cosmos. 1909;51:412–21. [Google Scholar]
- 3.Pussinen PJ, Alfthan G, Rissanen H, Reunanen A, Asikainen S, Knekt P. Antibodies to periodontal pathogens and stroke risk. Stroke. 2004;35:2020–3. doi: 10.1161/01.STR.0000136148.29490.fe. [DOI] [PubMed] [Google Scholar]
- 4.Sanz M, D’Aiuto F, Deanfield J, Francisco Fernandez-Aviles. European workshop in periodontal health and cardiovascular disease — Scientific evidence on the association between periodontal and cardiovascular diseases: A review of literature. Eur Heart J Suppl. 2010;12(Suppl B):B3–12. [Google Scholar]
- 5.Buhlin K, Gustafsson A, Pockley AG, Frostegård J, Klinge B. Risk factors for cardiovascular disease in patients with periodontitis. Eur Heart J. 2003;24:2099–107. doi: 10.1016/j.ehj.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Hamissi J, Shahsavarani MT, Hamissi H. A Comparison of Serum Lipid Profile between Periodontitis Patients and Healthy Individuals. Iran Red Crescent Med J. 2011;13:283–4. [PMC free article] [PubMed] [Google Scholar]
- 7.AL-Rawi NH, Atiyah KM. Assessment of salivary lipid profiles in patients with ischemic stroke and patients at risk of having stroke among Iraqi sample. Internet Journal of Third World Medicine. 2008;7:4. [Google Scholar]
- 8.Al-Rawi NH. Oxidative stress, antioxidant status and lipid profile in the saliva of type 2 diabetics. Diab Vasc Dis Res. 2011;8:22–8. doi: 10.1177/1479164110390243. [DOI] [PubMed] [Google Scholar]
- 9.Larsson B, Olivecrona IG, Ericson T. Lipids in human saliva. Arch Oral Biol. 1996;41:105–10. doi: 10.1016/0003-9969(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 10.Karjalainen S, Sewón L, Söderling E, Larsson B, Johansson I, Simell O, et al. Salivary cholesterol of healthy adults in relation to serum cholesterol concentration and oral health. J Dent Res. 1997;76:1637–43. doi: 10.1177/00220345970760100401. [DOI] [PubMed] [Google Scholar]
- 11.Tabak LA. A revolution in biomedical assessment: The development of salivary diagnostics. J Dent Educ. 2001;65:1335–9. [PubMed] [Google Scholar]
- 12.Kaufman E, Lamster IB. The diagnostic applications of saliva – A review. Crit Rev Oral Biol Med. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- 13.Taleghani F, Shamaei M, Shamaei M. Association between chronic periodontitis and serum lipid levels. Acta Med Iran. 2010;48:47–50. [PubMed] [Google Scholar]
- 14.Joshi NV, Marawar PP. Hyperlipidemia — A link between periodontitis and coronary heart disease. J Indian Dent Assoc. 2011;5:183–6. [Google Scholar]
- 15.Lösche W, Marshal GJ, Apatzidou DA, Krause S, Kocher T, Kinane DF. Lipoprotein-associated phospholipase A2 and plasma lipids in patients with destructive periodontal disease. J Clin Periodontol. 2005;32:640–4. doi: 10.1111/j.1600-051X.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 16.Iacopino AM, Cutler CW. Pathophysiological relationships between periodontitis and systemic disease: Recent concepts involving serum lipids. J Periodontol. 2000;71:1375–84. doi: 10.1902/jop.2000.71.8.1375. [DOI] [PubMed] [Google Scholar]
- 17.Noack B, Jachmann I, Roscher S, Sieber L, Kopprasch S, Lück C, et al. Metabolic diseases and their possible link to risk indicators of periodontitis. J Periodontol. 2000;71:898–903. doi: 10.1902/jop.2000.71.6.898. [DOI] [PubMed] [Google Scholar]


