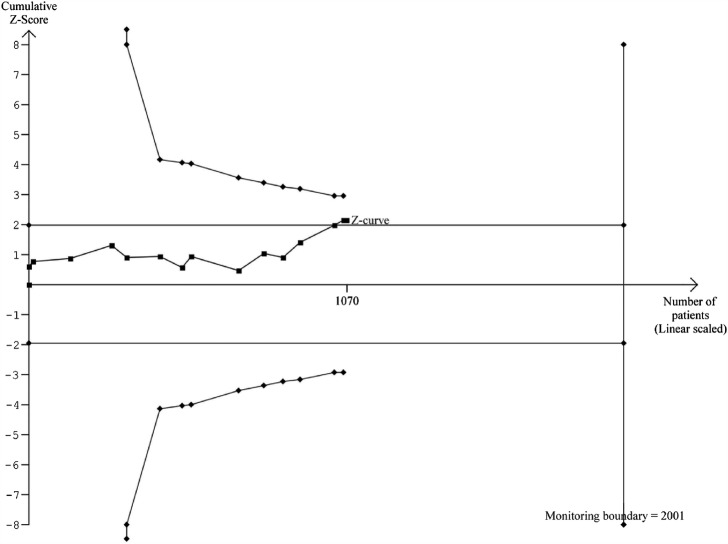
Figure 3.
Sequential analysis of risk ratios (random effects) in randomised controlled trials on lixisenatide versus placebo or active interventions for patients with type 2 diabetes and elevated alanine aminotransferase (ALT) at baseline. The analysis was performed with α 5%, power 80%, model-based diversity correction 12%, relative risk reduction 8% and control group incidence rate 51%. The outcome measure is normalisation of ALT. The analysis shows that lixisenatide has a beneficial effect on normalisation of ALT when assessed using the traditional 5% level of significance (the horizontal line), but not after adjusting for cumulative assessment (the trial monitoring boundary).