Abstract
INTRODUCTION
Cardiac perforation is a rare, but potentially serious, complication of pacemaker implantation that may develop days or weeks after implantation.
PRESENTATION OF CASE
In the current case, 92-year-old man underwent permanent pacemaker implantation, but he presented 3 weeks later with severe symptoms. Computed tomography showed protrusion of the tip of the ventricular electrode through the right ventricle and into the chest wall. During an urgent surgical intervention, the lead was disconnected and extracted. A sealing hemostatic device and an hemostatic patch were applied to repair the ventricle; the procedure was uneventfull.
DISCUSSION
This case demonstrates how the correct diagnosis of ventricular perforation is crucial, and should be followed immediately by surgical planning.
CONCLUSION
The hemostatic patch is a valuable alternative to sutures in patients with thin and fragile ventricular wall, unable to undergo stitching.
Keywords: Pacemaker, Computed tomography, Hemothorax, Chest wall, Surgery
1. Introduction
Complications after implantation of anti-bradycardia or anti-arrhythmic devices are uncommon1 and include infection of the device pocket, bacterial endocarditis, subclavian vein thrombosis, deficits in sensing or pacing, and pneumothorax.1,2 Cardiac perforation is a rare and dramatic event that occurs in 0.1–0.8% of cases3 primarily involving the right atrium, right ventricle and great veins.4 Perforation of the atrial or ventricular wall may be a risk for cardiac tamponade. In rare cases, perforation occurs hours, days or even weeks after implantation,2 with diagnosis potentially delayed due to the hemodynamic stability of the patient and the absence of symptoms.
2. Presentation of case
A 92-year-old man underwent dual mode, dual chamber, dual sensing (DDD) permanent pacemaker implantation for sick sinus syndrome and intermittent atrioventricular block with episodes of dyspnea and pre-syncope. The procedure was uneventful. The atrial and ventricular catheters were advanced through the right subclavian vein, the atrial lead was positioned and anchored in right appendage, and the ventricular lead was fixed on the apex of the right ventricle.
Following implantation, computer evaluation of the pacemaker showed that catheter impedance, sensing threshold and atrial/ventricular capture values were all within normal limits. Postoperative chest X-ray revealed that the pacemaker leads were in a good position, with no pneumothorax. The patient was discharged the day after implantation in good overall condition and prescribed aspirin 100 mg once a day.
Three weeks later, the patient presented at our institution complaining of positional dyspnea and recurrent pre-syncope episodes. Blood tests revealed a hematocrit of 28.3% and hemoglobin level of 8.5 g/dl, indicating anemia. All other values were within the normal range. Chest radiography revealed an opacity in the inferior field of the left lung, and protrusion of the tip of the ventricular electrode through the right ventricle and into the left chest wall at the level of the eighth intercostal space. Furthermore, appreciable cutaneous ecchymosis was identified in the anterolateral thorax at the level of the fifth–sixth intercostal space, as well as low-level intercostal muscle contraction, in accordance with ventricular lead stimulation. Chest computed tomography confirmed the perforation of the right ventricular wall, and the absence of pericardial effusion (Fig. 1A). The patient was referred for urgent surgery.
Fig. 1.
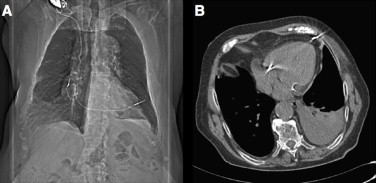
(A) Chest computed tomography showing lead perforation. (B) Computed tomography showing lead anchorage to chest wall.
As the unilateral opacity of the left chest observed on chest X-ray was interpreted as a massive pleural effusion, an attempt was made to drain the left pleural space.
Through a median sternotomy, the heart was exposed, which revealed evidence of a pacing wire protruding through the left ventricle close to the apex and penetrating the left pleura and thoracic wall (Fig. 1B). Although unclear on chest computed tomography, intraoperative examination suggested the lead had perforated the interventricular septum at the apex, via the left ventricle. Median sternotomy was preferred as the safest access approach owing to the age as well as general condition of the patient.
The ventricular lead was disconnected from the battery and extracted by pulling at the site of cannulation in the subclavian vein. The catheter tip was cut and the wire carefully pulled out. The left ventricle was repaired through application of sealing hemostatic devices (Floseal and Hemopatch; Baxter International, Deerfield, IL, USA), as shown in Fig. 2.
Fig. 2.
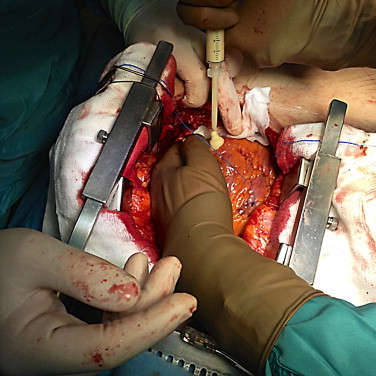
Application of Floseal to repair the left ventricle, before the application of Hemopatch.
The patient had an uneventful postoperative course. Echocardiogram revealed that the ventricle had been repaired successfully, with no ventricular shunt. The patient was discharged on postoperative day 5 day in good general condition.
3. Discussion
In the present case, cardiac perforation occurred approximately 3 weeks after implantation of the pacing device. The causes were unclear, but we can speculate that perforation resulted from the catheter loop exerting pressure on the right ventricle wall, which was thin owing to the patient's age. At the time of implantation, the ventricular lead was probably pushed too far into the right ventricle, considering that, after having perforated the ventricular wall, the catheter tip struck the chest wall with considerable force.
Perforation of the thoracic wall probably caused muscle bleeding, exacerbated by anti-platelet therapy. While this was self-limiting, it was sufficient to cause left hemothorax.
At the time of catheter extraction, sutures were not used due to the thin and fragile right ventricular wall. Consequently, after use of a hemostatic sealant, a hemostatic patch was applied directly to the ventricular perforation, resulting in total control of bleeding.
The hemostatic patch consists of a specially-formulated porous collagen matrix, coated on one side with a thin protein bonding layer of N-hydroxylsuccinimide (NHS) functionalized pentaerythritol polyethylene glycol ether tetra-succinimidyl glutarate (NHS-PEG). This gives the patch a dual mechanism of action, in which the two components interact to achieve hemostasis by sealing off the bleeding surface and initiating the patient's own clotting mechanism.
The use of hemostatic patch in cardiac surgery is largely supported by literature: Maisano et al.5 supported the use of TachoSil (Nycomed, Linz, Austria) to obtain better and fast intra-operative hemostasis in cardiovascular surgery. A cardiac post-infarction rupture repaired without stitches, with a tissue adhering patch is reported by Raffa and colleagues.6
Singhal et al.7 reported the case of late cardiac perforation by pacemaker lead 5 years after implantation. Other authors have reported similar cases, and it is remarkable that protrusion of a pacemaker lead through the ventricle may be diagnosed only on imaging. Chest X-ray is insufficient for accurate assessment of correct lead position. Although echocardiography may exclude pericardial effusion, in a few cases, such as ours, perforation of the heart does not cause effusion. Chest computed tomography remains the most accurate method of diagnosing lead perforation and establishing the exact position of the lead.
4. Conclusion
Late cardiac perforation by a ventricular lead is a rare but possible complication of pacemaker implantation. It may present days, weeks and sometimes even years after procedure. Follow-up examination of pacemaker function is essential, and must be made mainly by remote control as well as chest ray and echocardiogram. Correct diagnosis is crucial and requires appropriate examination. The only possible resolution is surgery, which must be planned immediately. For patients unsuitable for sutures, a hemostatic patch to cover the perforation and control bleeding is a valuable alternative.
An informed consent was obtained by the patient.
Conflict of interest
None declared.
Funding
None.
Ethical approval
Written informed consent was obtained from the patient.
Author contributions
Filippo Prestipino: study design; Antonio Nenna: writing; Adele Casacalenda: editing figures; Massimo Chello: supervisor of the clinical case.
Acknowledgement
The authors would like to thank Liam Davenport (Medicalwriters.com GmbH) for editorial support.
References
- 1.Piekarz J., Lelakowski J., Rydlewska A., Majewski J. Heart perforation in patients with permanent cardiac pacing – pilot personal observations. Arch Med Sci. 2012;8:70–74. doi: 10.5114/aoms.2012.27284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellenbogen K.A., Wood M.A., Shepard R.K. Delayed complications following pacemaker implantation. Pacing Clin Electrophysiol. 2002;25:1155–1158. doi: 10.1046/j.1460-9592.2002.01155.x. [DOI] [PubMed] [Google Scholar]
- 3.Koyama S., Itatani K., Kyo S. Subacute presentation of right ventricular perforation after pacemaker implantation. Ann Thorac Cardiovasc Surg. 2013;19:73–75. doi: 10.5761/atcs.cr.11.01863. [DOI] [PubMed] [Google Scholar]
- 4.Haque M.A., Roy S., Biswas B. Perforation by permanent pacemaker lead: how late can they occur. Cardiol J. 2012;19:326–327. doi: 10.5603/cj.2012.0059. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S., Cooper J.M., Cheung A.T., Acker M.A. Images in cardiovascular medicine Rib perforation from a right ventricular pacemaker lead. Circulation. 2007;115:e391–e392. doi: 10.1161/CIRCULATIONAHA.106.669630. [DOI] [PubMed] [Google Scholar]
- 6.Maisano F., Kjǽrgard H., Bauernschmitt R., Pavie A., Rabago G. Tachosil surgical patch versus conventional haemostatic fleece material for control of bleeding in cardio-vascular surgery: a randomized controlled trial. Eur J Cardiothorac Surg. 2009;36:708–714. doi: 10.1016/j.ejcts.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 7.Raffa G.M., Tarelli G., Patrini D., Settepani F. Sutureless repair for postinfarction cardiac rupture: a simple approach with a tissue-adhering patch. J Thorac Cardiovasc Surg. 2013;145:598–599. doi: 10.1016/j.jtcvs.2012.08.049. [DOI] [PubMed] [Google Scholar]


