Abstract
Although thrombus formation following myocardial infarction in adults is well known, intracardiac thrombosis in children is uncommon. We report the case of a large left ventricular thrombus in an infant with ischemic cardiomyopathy secondary to anomalous origin of the left coronary artery from the pulmonary artery. Given its mobility and protrusion across the aortic valve, the patient underwent urgent thrombus removal through a transaortic approach. There were no embolic or neurologic complications. This case highlights that thrombectomy may be performed safely and successfully in critically ill pediatric patients.
Keywords: Coronary artery anomaly, thrombosis (intracardiac), echocardiography, congenital heart surgery
Introduction
Intracardiac thrombosis in children is uncommon and has predominantly been described in patients with dilated cardiomyopathy or Fontan physiology.1,2 We report the case of an infant with a large, mobile intracardiac thrombus in the setting of ischemic cardiomyopathy secondary to anomalous origin of the left coronary artery from the pulmonary artery (ALCAPA) and describe our approach to its removal to prevent embolic complications.
Case Report
A five-week-old infant presented with tachypnea and poor pulses, which rapidly progressed to decompensated cardiogenic shock. Transthoracic echocardiogram (TTE) demonstrated a severely dilated left ventricle (LV), with end-diastolic dimension 3.7 cm (Z score +8.0) and end-systolic dimension 3.1 cm (Z score +12.1), and severely depressed LV function with ejection fraction (EF) 25% and shortening fraction (SF) 16%. In addition, echobright papillary muscles and mitral regurgitation were noted. Anomalous origin of the left coronary artery from the pulmonary artery was suspected, which was confirmed by aortic root angiography.
The patient was immediately taken to the operating room for surgical repair with direct reimplantation of the left main coronary artery to the aorta. Intraoperative transesophageal echocardiogram (TEE) demonstrated prograde flow in the left coronary artery by color Doppler. However, due to severe left ventricular dysfunction and inability to wean from cardiopulmonary bypass, the patient was placed on extracorporeal membrane oxygenation (ECMO).
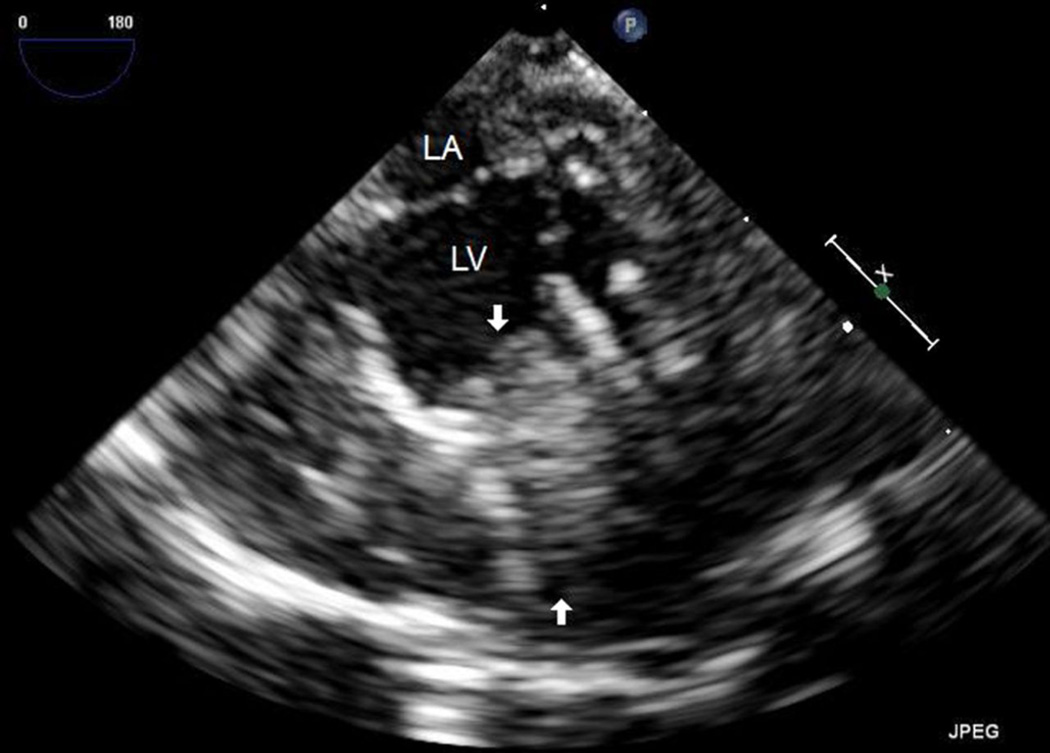
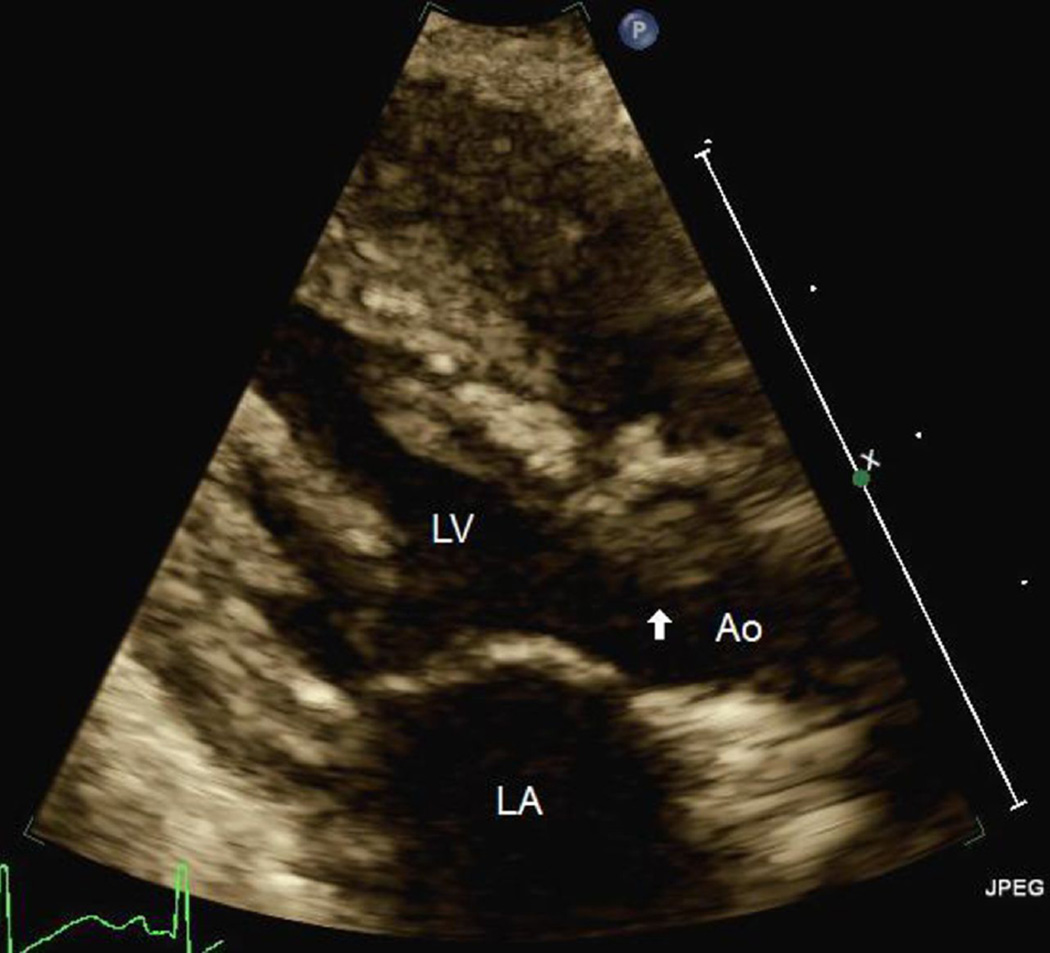
Repeat TEE at the time of decannulation from ECMO two days later demonstrated a new, large left ventricular thrombus adherent to the mid-septal wall (Figure 1). At that time, the LV end-diastolic and end-systolic dimensions had improved to 1.6 and 1.3 cm, respectively, with EF 32% and SF 19%. The patient had been maintained on a continuous heparin infusion with therapeutic activated clotting times (ACTs) between 180 and 200 seconds for the duration of mechanical support per our institution’s protocol. On postoperative day ten, despite continuation of systemic heparinization, TTE showed the thrombus to be increasingly mobile and protruding across the aortic valve in systole (Figure 2; Supplementary Data Video 1).
Figure 1.
The TEE image depicting a large thrombus in the LV (arrows), adherent to the interventricular septum.
Figure 2.
TTE image demonstrating the thrombus (arrow) crossing the aortic valve. Ao indicates aorta; LA, left atrium; LV, left ventricle; TTE, transthoracic echocardiogram.
Due to concern for systemic embolization and stroke, the patient returned to the operating room for removal of the thrombus. Preoperative TEE is shown (Supplementary Data Video 2). An aortotomy was performed, and a loose, friable thrombus partially adherent to the interventricular septum was identified and removed with suction and blunt dissection.
The patient had no evidence of distal embolization or neurologic complications during her postoperative course. In light of the successful thrombectomy and improving left ventricular function with EF 50% and SF 38% at the time of discharge, anticoagulation therapy was discontinued and aspirin was initiated for antiplatelet activity alone. At most recent follow-up two years later, the patient had a normal neurodevelopmental examination, with complete recovery of left ventricular function and no residual thrombus by TTE.
Discussion
We report the first case, to our knowledge, of a large, mobile left ventricular thrombus protruding across the aortic valve in an infant with severe ischemic cardiomyopathy secondary to ALCAPA. The patient’s course was complicated by significant left ventricular dysfunction requiring postoperative recovery on ECMO, underscoring the severity of her illness upon presentation. With full ECMO support, the poorly contractile LV was unable to eject, compounding the issue of stasis and accelerating thrombus formation despite systemic heparinization. Both TEE and TTE proved to be useful tools for diagnosing and serially evaluating the thrombus.
There is a paucity of literature regarding pediatric intracardiac thrombosis, most of which pertains to patients with dilated cardiomyopathy or Fontan physiology.1,2 Anomalous origin of the left coronary artery from the pulmonary artery, however, is a distinct disease process from most pediatric cardiac conditions that cause significant ventricular dysfunction and/or stasis in that it more closely resembles the model of myocardial infarction (MI) in adults. In particular, it mirrors a large, anterior myocardial infarction with apical hypokinesis, which is known to be associated with greater thromboembolic potential.3 In the post-MI state, not only do ventricular dysfunction and stasis contribute to an increased risk of thrombus formation, but also ischemia and myocardial necrosis lead to endothelial dysfunction and elaboration of inflammatory cytokines and procoagulant factors.4 This latter process, less well recognized by pediatric cardiac care providers, should prompt greater evaluation for thromboembolic phenomenon in ALCAPA case with severe LV dysfunction.
The mobility and protrusion of the thrombus across the aortic valve with each systolic contraction was unusual and deemed high risk for embolic complications based on findings from the adult literature.3 Furthermore, anticoagulation has not been shown to effectively prevent thromboembolism in this setting.5 There is minimal available data regarding the use of thrombolytic agents for pediatric intracardiac thrombosis in full-term infants, and a recent case series demonstrated a significant risk of major, and even fatal, bleeding complications.6 Meanwhile, surgical thrombectomy in adults has been feasible and successful, even in the early postinfarction period.7 We elected to perform urgent thrombectomy through a transaortic approach.
Postoperatively, we continued anticoagulation until ventricular function substantially improved (EF 50%), at which time we transitioned to aspirin. The most recent guidelines regarding antithrombotic therapy in children do not provide recommendations regarding the management of intracardiac thrombosis, with or without thrombectomy, likely owing to its rare occurrence.8 Therefore, we chose a conservative approach, extrapolating from the adult literature.
In summary, this case highlights that thrombectomy may be safely and successfully performed in pediatric patients with high-risk thrombi. In conjunction with conservative antithrombotic management, embolic complications may be avoided with excellent postoperative and neurologic outcomes.
Supplementary Material
Acknowledgements
This study was supported in part by National Institutes of Health under award number: T32HL007572. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms
- ACT
activated clotting times
- ALCAPA
anomalous origin of the left coronary artery from the pulmonary artery
- ECMO
extracorporeal membrane oxygenation
- EF
ejection fraction
- LV
left ventricle
- MI
myocardial infarction
- SF
shortening fraction
- TEE
transesophageal echocardiogram
- TTE
transthoracic echocardiogram
Footnotes
Declaration of Conflicting Interests:
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.McCrindle BW, Karamlou T, Wong H, et al. Presentation, management and outcomes of thrombosis for children with cardiomyopathy. Can J Cardiol. 2006;22(8):685–690. doi: 10.1016/s0828-282x(06)70937-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John JB, Cron SG, Kung GC, Mott AR. Intracardiac thrombi in pediatric patients: presentation profiles and clinical outcomes. Pediatr Cardiol. 2007;28(3):213–220. doi: 10.1007/s00246-005-1068-3. [DOI] [PubMed] [Google Scholar]
- 3.Visser CA, Kan G, Meltzer RS, Dunning AJ, Roelandt J. Embolic potential of left ventricular thrombus after myocardial infarction: a two-dimensional echocardiographic study of 119 patients. J Am Coll Cardiol. 1985;5(6):1276–1280. doi: 10.1016/s0735-1097(85)80336-3. [DOI] [PubMed] [Google Scholar]
- 4.Anzai T, Yoshikawa T, Kaneko H, et al. Association between serum C-reactive protein elevation and left ventricular thrombus formation after first anterior myocardial infarction. Chest. 2004;125(2):384–389. doi: 10.1378/chest.125.2.384. [DOI] [PubMed] [Google Scholar]
- 5.Lee JM, Park JJ, Jung HW, et al. Left ventricular thrombus and subsequent thromboembolism, comparison of anticoagulation, surgical removal, and antiplatelet agents. J Atheroscler Thromb. 2013;20(1):73–93. doi: 10.5551/jat.13540. [DOI] [PubMed] [Google Scholar]
- 6.Al-Jazairi AS, Al-Gain RA, Bulbul ZR, Cherfan SJ. Clinical experience with alteplase in the management of intracardiac and major cardiac vessels thrombosis in pediatrics: a case series. Ann Saudi Med. 2010;30(3):227–232. doi: 10.4103/0256-4947.62840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simolinsky A, Ziskind Z, Mohr R, Goor DA, Motro M. Left ventricular thrombectomy in the early post-infarction period. Thorax. 1990;45(7):548–551. doi: 10.1136/thx.45.7.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monagle P, Chan AKC, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 suppl):e737S–e801S. doi: 10.1378/chest.11-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.