Abstract
In recent years, the attachment mechanism of the octopus sucker has attracted the interest of scientists from different research areas, including biology, engineering, medicine and robotics. From a technological perspective, the main goal is to identify the underlying mechanisms involved in sucker attachment for use in the development of new generations of artificial devices and materials. Recently, the understanding of the morphology of the sucker has been significantly improved; however, the mechanisms that allow attachment remain largely unknown. In this work, we present new anatomical findings: specifically, a protuberance in the acetabular roof in five different octopus species; previously, this protuberance was identified by the authors in Octopus vulgaris. Moreover, we discuss the role of the protuberance and other anatomical structures in attachment with minimal energy consumption.
Keywords: octopus suckers, attachment, bioinspiration
1. Introduction
The octopus has been studied extensively. Several aspects of its biology have been investigated, including behaviour [1–3], sensory systems [4–7], nervous system [8,9], camouflage ability [10], self-recognition capability [11] and biomechanical features [12]. However, the ability of the octopus to attach to almost any object or surface with its suckers remains poorly understood. This lack of knowledge is largely for two reasons: the logistical constraints of investigating the attachment process in vivo and the lack of information on sucker anatomy and morphology. Recently, a promising solution to the former problem was proposed [13] that involved the use of ultrasonography to image the octopus tissues that are involved in the attachment process. The spatial resolution of this methodology is not very high; however, ultrasonography is non-invasive and allows in vivo observations of sucker morphology and how it interacts with objects and/or surfaces. This technology has been validated in Octopus vulgaris and has the potential to be successfully applied to other octopus species. In recent years, several studies have been conducted on sucker anatomy and morphology, helping to address the previous lack of information and identifying several unique traits [13–15]. In this work, we briefly describe the gross morphology of octopus suckers based on the available literature, present the first histological analyses of the suckers of several octopus species and discuss the proposed mechanism of attachment of the octopus sucker: the protuberance in the acetabular roof discovered in all the investigated octopus could be the key to realizing a smart and energy-efficient attachment.
2. Material and methods
Proximal suckers were extracted from 10 adult animals (two for each of the following species: Octopus maya, Octopus aegina, Thaumoctopus mimicus, Eledone cirrhosa and Eledone moschata) that had been anaesthetized and euthanized (by immersion for 30 min in 2 l of anaesthetic solution: 3.5% MgCl2 in seawater [16]). The suckers were fixed for 48 h in 4% paraformaldehyde (in 0.1 M phosphate buffer, pH 7.6, osmolarity controlled) in seawater at room temperature. They were then dehydrated in ethanol and embedded in paraffin. Suckers were serially sectioned (7-µm-thick sections) by a sliding microtome (Leica SM2010R) along the transversal plane. Sections were stained with Picro-Ponceau stain following Kier [17]. The sections were examined using an optical digital microscope (HIROX KH-7700).
3. Octopus sucker anatomy
The octopus sucker is a muscular hydrostat [18] composed of three types of muscular bundles: radial, circular and meridional. The radial fibres extend throughout the sucker wall, the circular fibres are arranged concentrically around the opening surface of the infundibulum and the meridional fibres radiate outwards from the apex (the proximal region of octopus sucker, where it embeds into the arm) downwards along the entire sucker (figure 1a). The sucker consists of two components: the infundibulum and the acetabulum. The infundibulum is the externally visible disc-like portion, whereas the acetabulum is embedded in the octopus arm and consists of an acetabular roof and an acetabular wall (figure 1a). Both the acetabulum and infundibulum are characterized by three types of muscular fibres with the exception of the acetabular roof, which lacks circular fibres and presents a random distribution of cross-connective fibres. The infundibulum and acetabulum communicate via an orifice (indicated by ‘o’ in figure 1a). The infundibular surface is characterized by radial and circumferential grooves and is completely encircled by an epithelial rim (‘rim’ in figure 1a) [13,14,19,20]. The structure of the acetabulum has long been debated. Nixon & Dilly [21] studied the sucker surface of several octopuses and described differences in acetabular morphology with respect to feeding habits and habitats. Their study is qualitative without quantitative comparisons. As demonstrated by Schmidtberg [22], the structure and sensory network of octopus suckers differ significantly between mature and immature suckers. The mature suckers are located along the proximal portion of the arm in adult animals and are primarily used for attachment. By contrast, immature suckers are present in both newly hatched animals and along the distal portion of the arm in adult animals and are largely involved in sensory functions; in fact, the octopus generally explores its environment using its arm tips. To properly compare sucker morphology among different species, suckers from the same region of the arm and from animals of similar age should be used. To this end, an octopus sucker identification code, which allows univocally the identification of suckers, was recently published [23]. Unfortunately, the sucker types in Nixon & Dilly [21] are unclear, precluding full comparisons. In contrast to Dilly and Nixon's study, subsequent works described the acetabulum as a spherical hollow structure with a completely smooth surface, with no differences among species [19,20]. Recently, to investigate this discrepancy, the sucker morphology of O. vulgaris was analysed using three different techniques: histology, magnetic resonance imaging and ultrasonography [13]. All three techniques show that, in O. vulgaris, the acetabulum of the proximal sucker presents an ellipsoidal hollow structure with a protuberance that fills almost the entire acetabular internal volume. In addition, a subsequent study [15] to investigate the acetabular surface of O. vulgaris, which was previously considered to be completely smooth, showed that the surface of the acetabular protuberance is densely covered by micro-hairs. In this study, we investigated whether the acetabular protuberance is unique to O. vulgaris by conducting histological analysis on different octopus species (O. maya, O. aegina, T. mimicus, E. cirrhosa and E. moschata). We found that the proximal suckers of adults in all species analysed showed a protuberance on the acetabular roof (figure 1b–f). These unexpected findings are highly relevant to the putative mechanism of sucker attachment.
Figure 1.
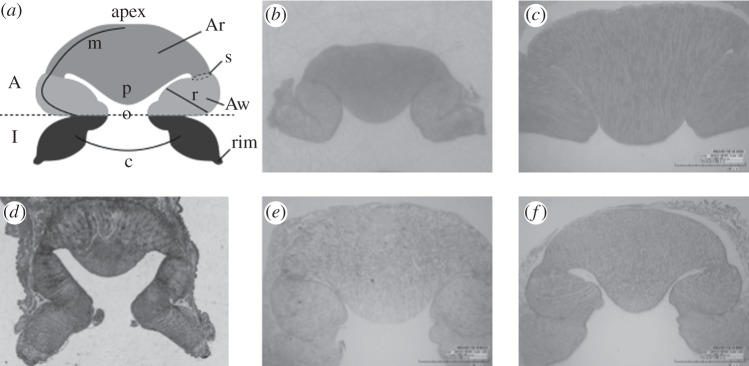
Structures of the octopus sucker. (a) Schematic representation: acetabulum (A); infundibulum (I); acetabular wall (Aw); acetabular roof (Ar); orifice (o); acetabular protuberance (p); rim (rim); orientation of meridional, circular and radial muscular fibres (m), (c) and (r), respectively; and strictures (s). (b–f) Transversal histological sections (7 µm thick) of (b) Octopus aegina; (c) Octopus maya; (d) Thaumoctopus mimicus; (e) Eledone cirrhosa; and (f) Eledone moschata, stained with Picro-Ponceau.
4. Discussion
Octopus vulgaris is a oceanodromous species and occurs widely throughout the world's oceans. This animal is generally reef-associated and inhabits depths of 0–200 m, although it is usually found at 0–100 m. The sucker morphology of this species has been widely investigated. Unlike other species of octopus studied, its sucker presents an unusual acetabular structure (acetabular protuberance) [13]. In this work, as previously mentioned, five different octopus species from different habitats were studied for the presence of acetabular structures. Octopus maya is a benthic species (the benthic zone is the lowest level of a sea or an ocean) that lives in the western central Atlantic Ocean at depths of 10–100 m in rocky and seagrass bottoms. Octopus aegina is generally found in the demersal zone (the zone closest to the sea floor) in the Indo-West Pacific Ocean at depths of 30–120 m. This small octopus lives on sand and muddy sea floors in coastal waters. Thaumoctopus mimicus also lives in the demersal zone of the Indo-West Pacific Ocean. Being a mimic octopus, it inhabits muddy river bottoms and estuaries at 15 m depth, where it can easily camouflage itself against the sea floor. Eledone cirrhosa lives in the demersal zone of the northeast Atlantic Ocean and Mediterranean Sea at depths of 0–770 m (more commonly 50–300 m) in muddy and sandy bottoms. Finally, E. moschata is found in the Mediterranean Sea and adjoining regions of the North Atlantic Ocean, usually at depths of 10–100 m, although it occasionally reaches depths of up to 300 m in some regions. This species generally prefers muddy bottoms, but it is also found on sand or gravel bottoms and, uncommonly, among rocks. Despite their varying habitats, all five octopus species investigated in this work exhibit the protuberance on the acetabular roof, as recently discovered in O. vulgaris. Therefore, the acetabular protuberance is not unique to O. vulgaris as previous hypothesized, but appears to be a common and thus robust feature of mature suckers in adult animals, unrelated to habitat conditions.
To date, the mechanism of octopus sucker attachment remains poorly understood. However, it is widely recognized that the infundibulum is first in contacting a substrate, and the acetabulum is responsible for performing the subsequent efficient attachment. Different hypotheses of the mechanism have been proposed. Girod [24] postulated that attachment occurs through the collapse of the entire acetabular chamber. In this scenario, the sucker remains fastened to substrate via the void created by the structural collapse. According to this hypothesis, the octopus would perform a type of passive attachment by forcing out the sucker's internal water volume. By contrast, detachment would be accomplished by rim lifting, allowing water to enter and fill the acetabular cavity. However, this proposed mechanism is flawed, as claimed by Guérin [25], who considered it imprecise and contradictory. In particular, Guérin [25] criticized the functional incoherence assigned to some muscular bundles, the description of the acetabulum as an elastic chamber that exhibits passive behaviour during the detachment phase, and, most importantly, the likelihood of achieving strong attachment. Moreover, this hypothesis does not address if or how the acetabular chamber improves attachment over a structure with only an infundibular portion (such as passive suction cups). In addition, according to Girod's hypothesis, the attachment strength would be limited to the vacuum, yet Smith [26] reports a lowest recorded pressure under the octopus sucker of 0.268 MPa below ambient. Therefore, Girod's proposed mechanism is inconsistent with the measurements of Smith in terms of the ability of the octopus sucker to generate negative pressure.
Kier & Smith [19] subsequently proposed a different sucker attachment mechanism. In their model, the sucker first attaches to substratum by forming a seal that prevents water from leaking in at the rim. Then, the sucker applies suction to maintain attachment, by reducing the pressure within the acetabular cavity (by contracting the acetabular radial muscles). The authors admit that this proposed mechanism is not energetically efficient because it requires that the octopus contracts its muscles throughout the entire attachment period (an unlikely scenario, considering that an octopus can remain attached to the substrate for hours; such an activity should involve minimal energy consumption). In an attempt to provide a rationale for this energetic requirement, the authors hypothesized that, during extended periods of attachment, the suckers maintain suction via a type of elastic strain energy storage within the acetabular cross-connective tissue [19,20]. Specifically, prior to attachment, the connective tissue fibres of the acetabular roof should be prestrained by the meridional and circumferential muscles (assumed to be antagonists of the radial muscles). Upon attachment, the stored strain energy might exert a force analogous to that created by the radial muscles [19]. Although this mechanism would represent a fascinating strategy for preserving energy, it does not comply with sucker anatomy as revealed in recent works (i.e. the presence and role of the acetabular protuberance are not addressed), and the posited role of the connective tissue has not been demonstrated.
The most recent hypothesis proposed in [13] is modified from Kier and Smith [19,20] but is more consistent with sucker morphology. The authors propose that attachment is performed in four steps (figure 2): (i) the infundibulum contacts the substrate, forming a seal; (ii) the acetabular radial muscles contract to generate suction; (iii) the acetabular meridional muscles contract (while the radial ones remain contracted), allowing the protuberance to contact the upper surface of the side wall of the orifice (such contraction is suggested by the strictures present at the interface of the acetabular roof and acetabular wall; see ‘s’ in figure 1a); and (iv) all muscles (both radial and meridional) cease contracting, with attachment guaranteed by the balance between the restoration of elastic force in the acetabular roof (see the white arrow in figure 2e) and the cohesive force of the water in the infundibular compartment (see the grey arrow in figure 2e), along with the attachment force exerted by the two surfaces in contact (the protuberance and the upper surface of the side wall of the orifice; see the black arrows in figure 2e). This hypothesis was first supported by the ultrasonographic recording conducted in the same work [13]. It has been further supported by the recent discovery of hair-like structures covering the entire surface of the acetabular roof [15]. Hair structures have been recognized as fundamental to attachment under wet conditions in other animals, such as clingfish and abalone molluscs [27,28]. This natural solution should prevent sliding between the acetabular protuberance and the orifice [29,30] and help to seal region Pi from region Pa. In this way, the water does not leak into the Pi region and the low pressure at the interface is maintained (see appendix A).
Figure 2.
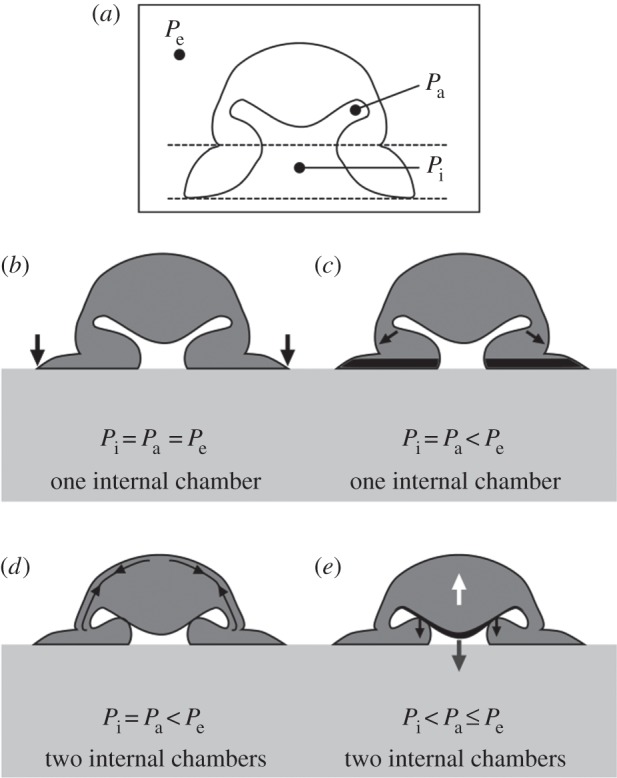
Hypothesis of octopus sucker attachment [13]. In all images, we can identify the pressure of three compartments: Pe, environmental pressure; Pa, pressure in the acetabular compartment of the sucker; and Pi, pressure in the infundibular compartment of the sucker. (a) Sucker in the resting state. (b) First step of the attachment process. The sucker comes into contact with the substrate, and a seal is formed around the infundibulum (black arrows). During this first step, the three pressures (Pi, Pa and Pe) are equal. (c) Second step of the attachment process. The contraction of the acetabular radial muscles (black arrows) applies tension to the water volume in the sucker, inducing a reduction of internal pressure. The grooves present on the infundibular surface (see the black-marked area) allow the low pressure generated in the acetabulum to be distributed across almost the entire attachment area of the sucker. During this second step, the pressure Pi is equal to Pa because the two compartments are still connected via the orifice, but their pressure values are lower than Pe. (d) Third step of the attachment process. The contraction of the acetabular meridional muscles (black arrows) places the acetabular protuberance in contact with the upper surface of the side wall of the orifice, inducing orifice closure. At this step, Pi is still equal to Pa, and both are still lower than Pe, but two different compartments have been formed within the sucker, an acetabular one and an infundibular one. (e) Last step of the attachment process. As all muscles cease contracting, a restoring elastic force (white arrow) induces detachment of the acetabular protuberance from the upper surface of the side wall of the orifice. To maintain attachment at the substrate without muscular force, the restoring elastic force is balanced by the cohesive force of the water within the infundibular compartment (the water volume behaves like a solid under tension; see the grey arrow), and the adhesion force (black arrows) exerted by the dense network of hairs (see the black arrows) is present on the acetabular protuberance's surface. As such, the forces are in equilibrium, and the sucker can maintain the low pressure at the interface without further energy consumption.
5. Conclusion
In conclusion, consistent with this most recent model of attachment [13,15], we speculate on the role of the cross-connective fibres. The cross-connective fibres embedded in the acetabular roof might work in a manner similar to that of fibres in composite materials; namely, by strengthening the overall structure. When the acetabular radial muscles contract to induce suction, only the radial fibres of the acetabular wall react, whereas the random cross-connective fibres prevent the contraction of the radial muscles in the acetabular roof (where the protuberance is located). At this stage, preserving the protuberance's configuration close to the orifice, minimal contraction of the acetabular meridional muscles causes the protuberance to contact the upper surface of the side wall of the orifice (the closure of the orifice). Finally, our hypothesized involvement of the acetabular protuberance in the attachment process is further supported by the presence of sensor receptors only on the surface of the acetabular roof (such receptors are completely absent from the acetabular wall) [31]. Graziadei [31] claimed that the receptors embedded in the acetabular roof are multi-polar nerve cells that belong to a category of sensory elements known as ‘tension receptors’. These types of receptors, located precisely in the region involved in orifice closure, might further assist in the attachment process.
Acknowledgement
The authors thank anonymous reviewers for their positive comments and very useful suggestions.
Appendix A. Adhesion constitutive law
Assuming that closure of the orifice is achieved through hair-based adhesion of the protuberances, we can estimate its mechanical behaviour via the peeling of a conical membrane; accordingly, we find [32] a force versus displacement dimensionless curve of type
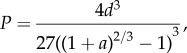 |
where P = F/(2πEr0t/(1 − v2)) is the dimensionless vertical force, F is the applied force, E is the Young modulus, v is the Poisson ratio, t is the sucker thickness, r0 is the protuberance radius, a = r/r0, where r is the sucker radius, and d = D/r0, where D is the vertical displacement.
The critical dimensionless condition for detachment is
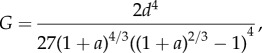 |
where G = 2g/(Et/(1 −v2)) and g is the surface energy per unit area of the protuberance.
Ethics statement
The studies were conducted in 2012. At that time, approval for research on these animals was not required, as European law did not require licensing until 2013. However, all research facilities and procedures complied with EU law (Directive 2010/63/EU) and the 3Rs (Replacement, Reduction and Refinement) rule (Directive 86/609/EEC).
Funding statement
This work was supported by the COST Action TD0906 ‘Biological adhesives: from biology to biomimetics’. N.M.P. is supported by the European Research Council (ERC StG Ideas 2011 BIHSNAM no. 279985 on ‘Bio-inspired hierarchical super-nanomaterials’, ERC PoC 2013–1 REPLICA2 no. 619448 on ‘Large-area replication of biological anti-adhesive nanosurfaces’, ERC PoC 2013-2 KNOTOUGH no. 632277 on ‘Super-tough knotted fibres’), by the European Commission under the Graphene Flagship (WP10 ‘Nanocomposites’, no. 604391) and by the Provincia Autonoma di Trento (‘Graphene Nanocomposites’, no. S116/2012-242637 and reg. delib. no. 2266).
Conflict of interests
The authors declare that there are no conflicts of interests.
References
- 1.Byrne RA, Kuba MJ, Meisel DV, Griebel U, Mather JA. 2006. Does Octopus vulgaris have preferred arms? J. Comp. Psychol. 120, 198–204. ( 10.1037/0735-7036.120.3.198) [DOI] [PubMed] [Google Scholar]
- 2.Gutnick T, Byrne RA, Hochner B, Kuba M. 2011. Octopus vulgaris uses visual information to determine the location of its arm. Curr. Biol. 21, 460–462. ( 10.1016/j.cub.2011.01.052) [DOI] [PubMed] [Google Scholar]
- 3.Mather JA, Kuba MJ. 2013. The cephalopod specialties: complex nervous system, learning, and cognition. Can. J. Zool. 91, 431–449. ( 10.1139/cjz-2013-0009) [DOI] [Google Scholar]
- 4.Graziadei P. 1962. Receptors in the suckers of octopus. Nature 195, 57–59. ( 10.1038/195057a0) [DOI] [Google Scholar]
- 5.Graziadei P, Gagne HT. 1976. Sensory innervation in the rim of the octopus sucker. J. Morphol. 150, 639–679. ( 10.1002/jmor.1051500304) [DOI] [PubMed] [Google Scholar]
- 6.Graziadei P, Gagne HT. 1976. An unusual receptor in the octopus. Tissue Cell 8, 229–240. ( 10.1016/0040-8166(76)90049-5) [DOI] [PubMed] [Google Scholar]
- 7.Messenger JB. 1979. The eyes and skin of octopus: compensating for sensory deficiencies. Endeavour 3, 92–98. ( 10.1016/0160-9327(79)90096-6) [DOI] [Google Scholar]
- 8.Alupay JS, Hadjisolomou SP, Crook RJ. 2014. Arm injury produces long-term behavioral and neural hypersensitivity in octopus. Neurosci. Lett. 558, 137–142. ( 10.1016/j.neulet.2013.11.002) [DOI] [PubMed] [Google Scholar]
- 9.Hochner B. 2008. Octopuses. Curr. Biol. 18, 897–898. ( 10.1016/j.cub.2008.07.057) [DOI] [PubMed] [Google Scholar]
- 10.Messenger JB. 1974. Reflecting elements in cephalopod skin and their importance for camouflage. J. Zool. 174, 387–395. ( 10.1111/j.1469-7998.1974.tb03166.x) [DOI] [Google Scholar]
- 11.Nesher N, Levy G, Grasso FW, Hochner B. 2014. Self-recognition mechanism between skin and suckers prevents octopus arms from interfering with each other. Curr. Biol. 24, 1271–1275. ( 10.1016/j.cub.2014.04.024) [DOI] [PubMed] [Google Scholar]
- 12.Mazzolai B, Margheri L, Cianchetti M, Dario P, Laschi C. 2012. Soft-robotic arm inspired by the octopus: II. From artificial requirements to innovative technological solutions. Bioinspir. Biomim. 7, 025005 ( 10.1088/1748-3182/7/2/025005) [DOI] [PubMed] [Google Scholar]
- 13.Tramacere F, Beccai L, Kuba M, Gozzi A, Bifone A, Mazzolai B. 2013. The morphology and adhesion mechanism of Octopus vulgaris suckers. PLoS ONE 8, e65074 ( 10.1371/journal.pone.0065074) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tramacere F, Kovalev A, Kleinteich T, Gorb SN, Mazzolai B. 2014. Structure and mechanical properties of Octopus vulgaris suckers. J. R. Soc. Interface 11, 20130816 ( 10.1098/rsif.2013.0816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tramacere F, Appel E, Mazzolai B, Gorb SN. 2014. Hairy suckers: the surface microstructure and its possible functional significance in the Octopus vulgaris sucker. Beilstein J. Nanotechnol. 5, 561–565. ( 10.3762/bjnano.5.66) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messenger JB, Nixon M, Ryan KP. 1985. Magnesium chloride as an anaesthetic for cephalopods. Comp. Biochem. Phys. C 82, 203–205. ( 10.1016/0742-8413(85)90230-0) [DOI] [PubMed] [Google Scholar]
- 17.Kier WM. 1992. Hydrostatic skeletons and muscular hydrostats. In Biomechanics (structures and system): a practical approach (ed. Biewener AA.), pp. 205–231. New York, NY: IRL Press. [Google Scholar]
- 18.Kier WM, Smith KK. 1985. Tongues, tentacles and trunks: the biomechanics of movement in muscular-hydrostats. Zool. J. Linn. Soc. 83, 307–324. ( 10.1111/j.1096-3642.1985.tb01178.x) [DOI] [Google Scholar]
- 19.Kier WM, Smith AM. 1990. The morphology and mechanics of octopus suckers. Biol. Bull. 178, 126–136. ( 10.2307/1541971) [DOI] [PubMed] [Google Scholar]
- 20.Kier WM, Smith AM. 2002. The structure and adhesive mechanism of octopus suckers. Integr. Comp. Biol. 42, 1146–1153. ( 10.1093/icb/42.6.1146) [DOI] [PubMed] [Google Scholar]
- 21.Nixon M, Dilly PN. 1977. Sucker surfaces and prey capture. Symp. Zool. Soc. Lond 38, 447–511. [Google Scholar]
- 22.Schmidtberg H. 1999. Ultrastructural studies of the suckers of newly hatched Eledone Moschata and Octopus Vulgaris (Mollusca; Cephalopoda). In Advancing research on living and fossil cephalopods (eds Olóriz F, Rodríguez-Tovar F.), pp. 203–221. New York, NY: Springer. [Google Scholar]
- 23.Tramacere F, Beccai L, Kuba MJ, Mazzolai B. 2013. Octopus suckers identification code (OSIC). Mar. Freshw. Behav. Physiol. 46, 447–453. ( 10.1080/10236244.2013.856586) [DOI] [Google Scholar]
- 24.Girod P. 1884. Recherches sur la peau des céphalopodes—La ventouse. Arch. Zool. Exp. Gen. 2, 379–401. [Google Scholar]
- 25.Guérin J. 1908. Contribution à l’étude des systèmes cutané, musculaire et nerveux de l'appareil tentaculaire des cèphalopodes. Arch. Zool. Exp. Gen. 38, 1–178. [Google Scholar]
- 26.Smith AM. 1991. Negative pressure generated by octopus suckers: a study of the tensile strength of water in nature. J. Exp. Biol. 157, 257–271. [Google Scholar]
- 27.Bravo Portela I, Martinez-Zorzano VS, Molist-Perez I, Molist García P. 2012. Ultrastructure and glycoconjugate pattern of the foot epithelium of the abalone Haliotis tuberculata (Linnaeus, 1758) (Gastropoda, Haliotidae). Sci. World J. 2012, 960159 ( 10.1100/2012/960159) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wainwright DK, Kleinteich T, Kleinteich A, Gorb SN, Summers AP. 2013. Stick tight: suction adhesion on irregular surfaces in the northern clingfish. Biol. Lett. 9, 20130234 ( 10.1098/rsbl.2013.0234) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varenberg M, Gorb SN. 2009. Hexagonal surface micropattern for dry and wet friction. Adv. Mater. 21, 483–486. ( 10.1002/adma.200802734) [DOI] [Google Scholar]
- 30.Tsipenyuk A, Varenberg M. 2014. Use of biomimetic hexagonal surface texture in friction against lubricated skin. J. R. Soc. Interface 11, 20140113 ( 10.1098/rsif.2014.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Graziadei P. 1964. Receptors in the sucker of the cuttlefish. Nature 203, 384–386. ( 10.1038/203384a0) [DOI] [PubMed] [Google Scholar]
- 32.Afferrante L, Carbone G, Demelio G, Pugno N. 2013. Adhesion of elastic thin films: double peeling of tapes versus axisymmetric peeling of membranes. Tribol. Lett. 52, 439–447. ( 10.1007/s11249-013-0227-6) [DOI] [Google Scholar]


