Abstract
Glandular trichomes of the carnivorous plant Roridula gorgonias release a viscous resinous secretion. Its adhesion to hydrophilic and hydrophobic glass surfaces was measured in air and underwater. The underwater adhesion reached up to 91% (on hydrophilic glass) and 28% (on hydrophobic glass) of that measured in the air. After being submersed for 24 h in water, trichomes did not lose their ability to adhere to both types of glass surfaces underwater. We assume that acylglycerides and triterpenoids, which have been demonstrated previously to be main compounds of the secretion, cause the predominantly non-polar character and the insolubility in water. The robustness of the secretion to a wet environment presumably enables the plant to maintain its trapping function also under humid conditions and during rainy weather.
Keywords: glue, adhesive, biomechanics, glandular trichomes, plant surface, resin
1. Introduction
The viscid secretion released from glandular trichomes of the carnivorous flypaper plant Roridula gorgonias Planch. (Roridulaceae) [1–3] has been suggested to be the strongest and extremely effective glue among those evolved in insect-trapping plants because of the conspicuous number of observed trapped insects of considerable body size and mass [1,4–6]. Three different trichome types of different geometry and stiffness act together in the three-dimensional, hierarchical trap: (i) long tentacle-shaped, (ii) medium-sized, and (iii) short [3]. Prominent, long trichomes with less viscous secretion are responsible for establishment of initial contacts and entanglement of the prey, whereas the short trichomes, bearing more viscid secretion, completely immobilize it. For example, a 60 mg heavy fly, glued to a single short trichome, could only be removed with a pull-off force 4.8 times higher than the fly body weight [2].
The secretion is of resinous nature and mainly composed of triterpenoids (taraxeradiol, dihydroxyolean-12-ene, dihydroxyurs-12-ene, 3 unknown triterpenediols), acylglycerides (mono- and diacylglycerides) and traces of germanicol and 3α-lupeol [7]. No saccharides or proteins have been previously detected. The chemical composition of a residual secretion component remains unclear. Because the secretion extends into filaments during pulling on it, its elastomeric properties have been previously assumed [2,8,9].
The secretion drop is water-insoluble, maintaining ovoid or spherical shape after washing with water [8–11]. Because of its resistance to water, one may suggest that it can also adhere to substrates in an aquatic environment. The secretion may be periodically exposed to wet conditions, such as heavy rainfalls in the South African biome fynbos [12], to which R. gorgonias belongs. Water-soluble secretion, such as the case of carnivorous plants Drosera, Drosophyllum and Byblis [13,14], can be simply washed out by rain, which would require an additional secretory activity to reset the adhesive ability of the plant trap. Interestingly, underwater attachment in aquatic organisms is usually based on proteinaceous secretions (reviews by Scherge & Gorb [15] and Flammang [16]). Taking into account the general interest in underwater adhesion [17], the adhesive performance of the resinous plant secretion in the presence of water has to be examined.
In this study, the secretion of R. gorgonias was visualized in dry and aqueous environments using light microscopy techniques. Its adhesive properties in air and underwater on hydrophilic (normal) and hydrophobic (silanized) glass surfaces were measured using a cell load force transducer.
2. Material and methods
2.1. Plants
One- to three-year-old R. gorgonias were obtained from a private glasshouse culture (Klaus Keller, Augsburg, Germany), kept under laboratory conditions during experiments (23.7 ± 1.7°C, 47.3 ± 10.0% RH, 16 h photoperiod) and supplied with wingless adult fruit flies Drosophila melanogaster Meigen (Diptera, Drosophilidae). For experiments, short trichomes of the 10th–20th leaves below the apex were selected.
2.2. Microscopy
Microscopic observations of trichomes with adhesive secretion were conducted using a stereomicroscope Olympus SZX 12 with a DF PLAPO 1xPF objective (Olympus Corp., Tokyo, Japan). Images were taken using a Nikon Coolpix E995 digital camera adapted to the stereomicroscope with a C-Mount adapter and an MDC 2 relay lens MXA 29005 (Nikon Corp., Tokyo, Japan).
2.3. Force measurements
Using a razor blade, a piece of leaf tissue bearing a single short glandular trichome with a spherical secretion droplet of 0.25 mm diameter on its tip was cut out of a living leaf of R. gorgonias. The leaf sample, with the trichome oriented horizontally, was mechanically clamped with tweezers attached to a holder (figure 1a). Slides of normal and silanized glass (5 mm diameter, Superior, Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) were used as test substrates. They were glued perpendicularly to one end of a thin-walled glass capillary (1 mm diameter, TW100F-4, World Precision Instruments, Inc., Sarasota, FL) using super glue Loctite (Henkel Loctite Deutschland GmbH, München, Germany). Glass slides and capillary were cleaned prior to experiments by successive immersions in piranha solution (mixture of sulfuric acid (H2SO4) and hydrogen peroxide (H2O2), 3 : 1), rinsed with Aqua Millipore water and dried immediately by means of compressed air. To hydrophobize the glass surface, it was silanized with 1H,1H,2H,2H-perfluorodecyltrichlorosilane 97% (C10H4Cl3F17Si, SIH5841.0, ABCR GmbH & Co. KG, Karlsruhe, Germany). The quality of hydrophobization was tested using OCAH 200 contact angle measurement device (Dataphysics, Filderstadt, Germany; 1 µl of Aqua Millipore water on normal glass: 45.4 ± 2.29°; on silanized glass: 110.3 ± 1.11°; n = 10).
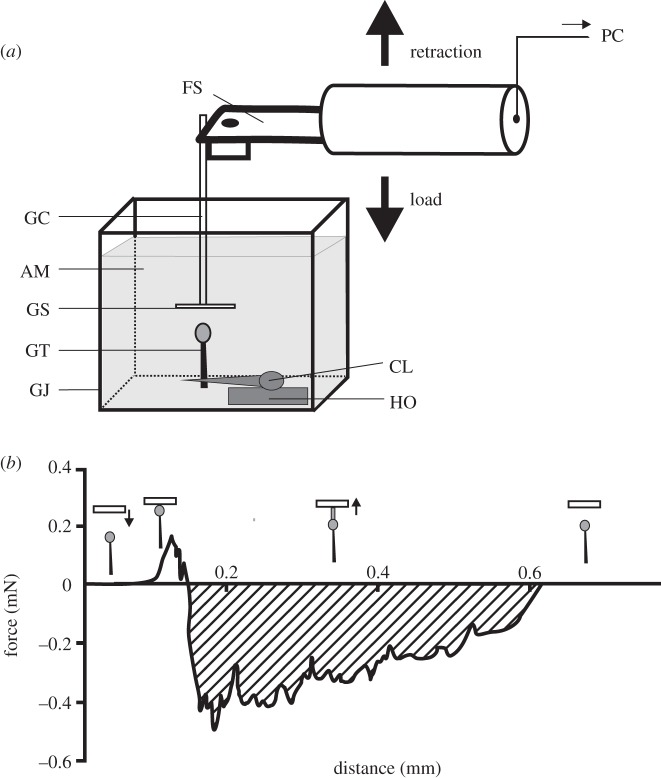
Figure 1.
Force measurements. (a) An experimental design for measuring underwater adhesion of single trichomes of R. gorgonias. The fresh trichome (GT) was perpendicularly clamped (CL) and mounted on a horizontal holder (HO). A force sensor (FS) with a firmly adhering glass capillary (GC) and attached glass slide (GS) was moved down, using a motorized micromanipulator, until the contact between the GS and the secretion of the GT was established. Then the sensor with the secretion adhering to the GS was pulled off. The force–time signal was recorded and processed further in a computer (PC). A glass aquarium (GJ) was filled with Aqua Millipore water (AM) during underwater measurements. (b) Representative force–distance curve, transformed from obtained force–time curve. It displays a transition at 0.2 mm which indicates that instabilities have developed in order to relieve the force. Insets show the trichome position relative to the glass slide at each part of the force–distance curve. Arrows point to the direction of the substrate movement. The shaded area indicates the work that had to be applied to retract the adhering trichome from the surface.
The free end of the capillary was firmly attached to a force transducer (10 g capacity, Biopac Systems Ltd, Santa Barbara, CA) combined with a motorized micromanipulator DC3314R and a controller MS314 (World Precision Instruments Inc.). The glass slide attached to the capillary tip was moved up and down with a velocity of about 30 µm s−1. The slide was brought into contact with the trichome head, preloaded, and then withdrawn. Force–time curves were recorded using AcqKnowledge v. 3.7.0 software (Biopac Systems, Inc., Goleta, CA) at an acquisition sample rate of 500 Hz, transformed to force–distance curves, from which the pull-off force was estimated (figure 1b).
Underwater experiments were carried out in an Aqua Millipore environment. For each substrate type (normal and silanized glass), n = 20 measurements with N = 20 single trichomes were performed at three different environmental conditions: in air, underwater and underwater using trichomes which were submersed in Aqua Millipore water for 24 h prior to measurements. In total, 120 tests were carried out.
For statistics, pull-off force values obtained on a single surface type at different conditions were compared using one-way ANOVA or Kruskal–Wallis one-way ANOVA on ranks, both followed by all pairwise multiple comparison procedure (Tukey's or Dunn's test) according to the distribution of the data. Differences between pull-off force values on normal and silanized glass surfaces were estimated using Mann–Whitney rank-sum test (SigmaStat v. 3.1.1 software, Systat Software Inc., Richmond, CA).
3. Results
3.1. Secretion in air and underwater
Secretion droplets on trichome tips (figure 2) appeared shiny and transparent in air. Their spherical shape remained stable underwater even after 24 h of submersion (figure 2a,c). However, droplets looked ‘milky’ underwater, and sometimes appeared spotted after being submersed (figure 2b,d,e). Complete dissolution was never observed.
Figure 2.
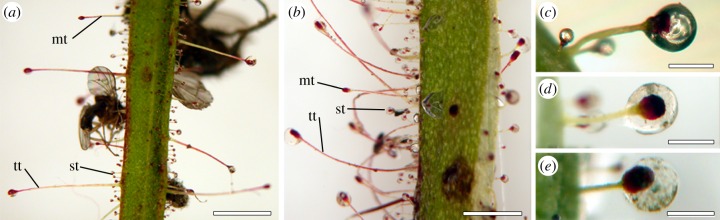
Glandular trichomes of R. gorgonias at different conditions. (a) Adaxial leaf side covered with trichomes in air. (b) Adaxial leaf side covered with trichomes in Aqua Millipore water. (c–e) Short glandular trichome in air (c), in water (d), after 24 h submersion in Aqua Millipore water (e). tt, long tentacle-shaped trichome; mt, medium-sized trichome; st, short trichome. Scale bars, (a,b) 2 mm, (c–e) 0.2 mm.
3.2. Adhesion
In all trials, adhesive failure was observed, i.e. plant secretion separated from the glass surface without leaving any residue.
The pull-off force required to retract an adhering trichome from the surface depended on the surface type and environmental conditions (figure 3 and table 1). On hydrophilic glass, no statistical differences in force between air and underwater environment were found. On hydrophobic glass, both types of underwater experiments resulted in rather low pull-off force compared with those measured in the aerial environment (28% immediately after submersion, and 26% after 24 h of submersion). In air, significantly higher pull-off force was required to pull the secretion from the hydrophobic surface than from the hydrophilic one. In both conditions, underwater and underwater after 24 h submersion, no significant difference was observed in measured pull-off force values between normal and silanized glass. Considering the secretion droplet diameter of 0.25 mm (cross-sectional area of 0.05 mm2), the adhesive strength (tenacity) underwater was estimated between 3.3 (hydrophilic glass) and 2.2 kPa (silanized glass).
Figure 3.
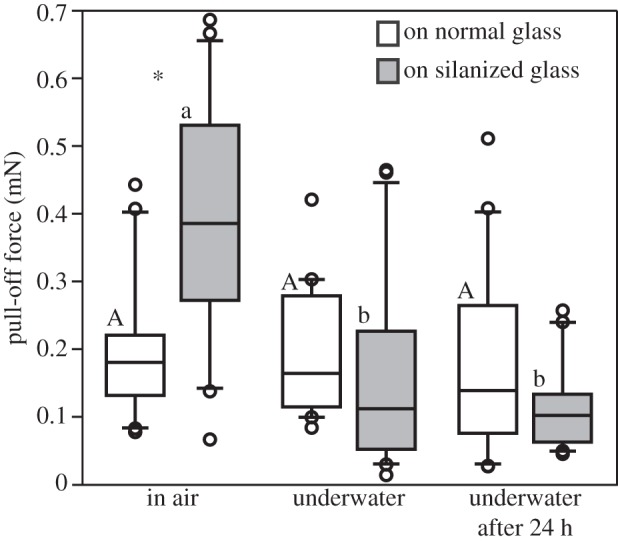
Box-and-whisker diagram of pull-off force required to break the contact between the trichome secretion and different glass surfaces at different conditions: in air, underwater, and underwater after 24 h of submersion. The ends of the boxes define the 25th and 75th percentiles, with a line at the median and error bars defining the 10th and 90th percentiles. Upper-case letters indicate statistical differences between different conditions on normal glass and lower-case letters on silanized glass, according to Kruskal–Wallis one-way ANOVA on ranks and Tukey's test, p < 0.05 (for normal glass: H2,57 = 2.4, p = 0.307; for silanized glass: H2,57 = 25.2, p ≤ 0.001). Asterisks show significant differences between normal and silanized glass at the same condition. For further statistics values, see table 1.
Table 1.
Statistical differences in pull-off force between normal and silanized glass according to Mann–Whitney rank-sum analysis. See asterisk in figure 3.
| condition | T-statistics | p-value | significance |
|---|---|---|---|
| in air | 741 | 0.041 | significant |
| in water | 436 | 0.156 | non-significant |
| in water after 24 h | 446 | 0.337 | non-significant |
Similar to experiments in air, the secretion was pulled into long thin filaments also underwater. The separation distance to interrupt the filament ranged from about 1452 µm on normal glass underwater after 24 h submersion to about 2260 µm on silanized glass underwater, without statistical difference between conditions on silanized glass (Kruskal–Wallis one-way ANOVA on ranks, H5,114 = 1.5, p = 0.472). On normal glass, filaments were statistically longer in air than in water after 24 h (Kruskal–Wallis one-way ANOVA on ranks, H5,114 = 10.5, p = 0.005 followed by all pairwise multiple comparison procedure, Tukey's test, p < 0.05). In the aqueous environment, filaments pulled off from silanized glass were significantly longer than those from normal glass; however, their length on both glass surfaces did not differ in air (table 2). The length of filaments (l) did not influence the measured pull-off force (Fp) (Fp = 0.20 + 0.0000477 × l, R2 = 0.01, F1,4 = 0.04, p = 0.85, linear-regression analysis).
Table 2.
Median length of filaments (minimum–maximum) into which plant secretion was pulled off during force measurements on different surfaces at various conditions, and statistical differences in filament length between secretion droplets pulled off from normal and silanized glass (Mann–Whitney rank-sum analysis).
| condition | length of filaments (µm) on |
statistical differences |
||
|---|---|---|---|---|
| normal glass | silanized glass | T-statistics | p-value | |
| in air | 2493.4 (864.2–3932.1) | 2125.8 (646.7–3897.3) | 452.5 | 0.256 non-significant |
| in water | 1081.3 (103.1–3676.2) | 2478.4 (1275.4–3897.4) | 322.0 | 0.018 significant |
| in water after 24 h | 1155.3 (285.4–3357.7) | 2740.9 (576.4–3632.4) | 329.5 | 0.030 significant |
The work required to retract the trichome from the glass surface was estimated from force–distance curves (figure 1b). It was significantly lower on normal (0.20 J) than on silanized glass (0.37 J) in air (Mann–Whitney rank-sum test, T = 426.5, p = 0.006), and significantly lower in water and 24 h under water than in air (Kruskal–Wallis one-way ANOVA on ranks followed by all pairwise multiple comparison procedure, Dunn's test (p < 0.05), for normal glass: H2,18 = 15.6, p ≤ 0.001; for silanized glass: H2,18 = 19.9, p ≤ 0.001). Underwater and 24 h underwater, the work did not differ statistically between normal (0.15, 0.08 J, respectively) and silanized glass (0.14, 0.09 J, respectively). Considering the influence of the length of filaments (l) on the estimated pull-off work (Wp), a slightly, but not significantly positive trend was observed (Wp = −0.04 + 0.0000983 × l, R2 = 0.16, F1,4 = 0.74, p = 0.44, linear-regression analysis).
4. Discussion
The secretion of R. gorgonias adhered well to normal and silanized glass in air and underwater. The pull-off force required to retract a short trichome from the hydrophobic glass surface in air was significantly higher than that on the hydrophilic glass [3]. This result may indicate certain secretion specialization in capturing insects usually having hydrophobic cuticle [18].
Wetting-depending adherence of glues has been previously discussed because of the complexity of influencing factors [19]. Supporting the data on recent studies of trichome adhesion to glass surfaces [3], the secretion of R. gorgonias adhered to normal and silanized glass in air and underwater. As anticipated, the pull-off force required to retract a short trichome from the glass surface differed between hydrophobic and hydrophilic glass surfaces. Surprisingly, glass silanization leads in air to an increase, but underwater to a decrease of the pull-off force. Below, we present a theoretical explanation of this effect. It is based on the assumption that the contact angle θ of the glue on the glass surface is within the interval 0° < θ < 90° and on the observation that the circumference C of the glue droplet on the glass at pull-off time remains always the same.
In air, the pull-off force F on normal glass is given by the expression Fa = σag C sinθ, and on silanized glass by Fa = σag C sinθ with σag denoting the surface tension σ acting on a droplet of plant glue placed on a glass surface under air (the indices a and g indicate air and plant glue, respectively). Similar expressions hold underwater (index w), if σag is replaced by σwg, and Fa by Fw.
In air, we observed Fa > Fa which implies sinθ > sinθ, then θ > θ, and thus cosθ < cosθ according to well-known properties of the sine and cosine function and to our assumptions on C and θ. Denoting changes of the surface tension owing to silanization by Δ and subtracting Young's equation [20] σag cosθ = σas − σsg for normal glass, from its version for silanized glass, σag cosθ = σas + Δσas − (σsg + Δσsg), we find σag cosθ − σag cosθ = Δσas − Δσsg < 0, hence Δσsg > Δσas (the index s stands for the solid, i.e. glass).
Underwater, the observation that Fw < Fw leads owing to similar reasoning to the result Δσws > Δσsg. Thus, the variations of pull-off forces, observed on the different combinations of normal/silanized glass versus air/water conditions, depicted in figure 3, are tantamount to the surface tension changes
| 4.1 |
produced by silanizing the glass. Our explanation of the unusual behaviour of the pull-off forces is based on these surface tension changes.
A partial justification for relation (4.1) derives from exploiting Young's equation once again: as mentioned above, Aqua Millipore behaves (in air environment) hydrophilic on normal glass, but hydrophobic on silanized glass, that is α > α, implying cos α < cos α, if α and α denote the respective contact angles. Proceeding as above, we obtain with Young's equation σwa cos α − σwa cos α = σas + Δσas − (σws + Δσws) − σas + σws = Δσas − Δσws < 0, hence
| 4.2 |
which is partial proof to (4.1). The meaning of this relation is that glass silanization affects the interaction between solid (glass) and water molecules more strongly than the interaction between solid and air molecules. Because water is polar, but air is not, and because adhesive secretions produced by various animals and plants seem to have both polar and non-polar properties, it is plausible to assume that the effect of silanization on the interaction between solid and glue is of a moderate magnitude as indicated by equation (4.1).
This study demonstrates for the first time that R. gorgonias secretion adheres underwater (figure 3). The appearance of long, thin, extensible and recoverable fluid filaments resulting from pulling the adhesive droplets in the aqueous environment supports previous assumptions about the viscoelastic property and strong cohesion of the secretion [2]. Extended force–distance curves, showing maximum initial peak and several following lower peaks (figure 1b), indicate large extension of secretion droplets into filaments (this effect is called fibrillation) and their previously discussed composite structure consisting of a fibrous network embedded in a fluid matrix [2]. This behaviour of secretion lets us suppose the presence of flows in the moving liquid (secretion) and did not differ in air and underwater. According to previous results, there is no significant influence of the length of filaments on the measured pull-off force [2]. Assuming the filaments as small springs glued to glass, having the same glued area, the force does not depend on how the springs are extended. Their extension will be different depending on stiffness. Spring deformation does not correlate with the glue strength. However, significantly longer filaments of secretion droplets suggest larger viscous dissipation, i.e. a higher internal friction of molecules in the secretion matrix and thus a strong resistance to separation, as on normal glass in air and on silanized glass in air, underwater and 24 h underwater. The filament length did not significantly correspond to the work required to retract the trichome from the glass surface; however, a slight positive trend was observed. In air, two times more work had to be applied to separate the secretion droplet from silanized glass (0.37 J) than from normal glass (0.20 J) and living blowfly Calliphora vicina Rob.-Des. (Diptera, Calliphoridae; 0.18 J), as was shown in previous studies [2]. Underwater, the work was similar for both glass types (normal: 0.15 J, silanized: 0.14 J), roughly corresponding to values obtained for blowflies [2]. After 24 h underwater, significantly lower work than in air and underwater had to be generated on both glasses (normal: 0.08 J, silanized: 0.09 J), resembling that previously estimated on the ‘sloughing-off’ surface of bugs Pameridea roridulae Reuter (Heteropera, Miridae; 0.07 J).
Roridula gorgonias secretion may attach in air and underwater on both polar and non-polar surfaces. That is why one may assume that adhesive secretion should be able to remove or adsorb boundary water layer from the surface of the solid substrate [17]. The major known components of the secretion of R. gorgonias are non-polar. For example, high-molecular-weight triterpenoids may cause its insolubility and water repellency. On the other hand, significant concentrations of mono- and diacylglycerides and their free OH-groups, found in the secretion of R. gorgonias [7], represent sites for possible strong polar interactions with hydrophilic substrates. In such structured fluids as plant secretions, monomers have affinity for a fluid boundary [21]. Monoglycerides are known to accumulate at oil–water or air–water interfaces, but not at oil–air interfaces, and smaller molecules of monoglycerides are slightly water-soluble [22]. These facts may explain the curious differences in pull-off forces required to retract the sticky plant secretion from hydrophobic and hydrophilic glass surfaces in air and underwater. Further, the certain solubility of monoglyceride molecules in water may explain the ‘milky’ appearance of plant secretion droplets underwater. Such ‘milky’ appearance is presumably owing to emulsification of external layers of the glue. A similar ‘milkiness’ underwater was observed for submersed adhesive tapes tesafilm (tesa SE, Hamburg, Germany) and Scotch Crystal and Scotch Magic (3M Deutschland GmbH, Neuss, Germany; D. Voigt 2010, unpublished data). Considering also previous observations on hygroscopic properties of R. gorgonias secretion [11], another reason may be swelling in the aqueous environment by ion interactions, as repeatedly reported for resins and elastic materials [23–25]. Most resins swell more strongly in polar than in less polar solvents, diminishing but not eliminating cohesion and becoming softer and flexible [24,26].
The adhesive strength of 2.2 kPa for R. gorgonias secretion droplets underwater is much lower than those reported for permanent adhering aquatic organisms. However, considering previous results separating a thin layer of R. gorgonias secretion between two glass slides [3], its effective adhesive strength may be expected about 15 times higher than the currently estimated values from pull-off forces necessary to detach secretion droplets on trichomes from glass. For example, compared with the plaque of the mussel Mytilus edulis L. (Mollusca, Mytiloidea, Mytilidae), adhering to a smooth glass surface (316 and 750 kPa), and the adult barnacle Balanus balanoides L. (Arthropoda, Cirripedia), adhering to a slate surface (930 kPa), the underwater adhesive strength of the plant secretion is 9–26 times lower [27–29]. It rather corresponds to that found in organisms having transitory underwater adhesion, for example 19 kPa in the beadled anemone Actinia equina L. (Anthozoa, Actiniidae) or 43 kPa in the plumose anemone Metridium senile L. (Anthozoa, Metridiidae; review by Scherge & Gorb [15]).
In contrast to the permanent nature of adhesion of proteinaceous aquatic glues [15,16], the secretion of R. gorgonias does not cure or dry out [11]. Recently, this glue has been shown to behave similarly to pressure-sensitive adhesives, in which resins are known as tackifiers [3,30]. Epoxy-based resin systems are commonly used as permanent adhesives for commercial applications in humid environments [31]. Thus, the secretion of R. gorgonias should be suitable for temporary adhesion, also in an aqueous environment. However, because the permanence of glue bonds is rather significant than their initial adherence [19], long-term studies on attachment performance should be subsequently undertaken to prove the reliability of Roridula glue.
Because rainwater cannot dissolve or dilute the secretion, the plant may not have to renew secretory fluid after each rain or morning dew. This might be important for the plant to maintain similar adhesive ability at various weather conditions in its habitat. Additionally, the use of persistent resinous secretion could save energy because of no need of resetting secretion after each rain and dry weather periods [11]. Such an adaptation clearly provides strong selective advantages of such an adhesive mechanism for the fynbos biome, if compared with the water-soluble glues found in species of the carnivorous flypaper plant genera Drosera, Drosophyllum or Byblis, growing in rather humid habitats [13,14]. Secretion of those plants has been reported to be aqueous solution of mucopolysaccharides [32,33]. One explanation why the polysaccharide strategy is evolutionarily stable is that the Drosera glue has only 4% of the solid substance (the rest is water) [33]. That is why the costs to be washed out by rain are not that high, and the properties can be quickly re-established after rain. But in the case of 100% of non-water-based glue, the loss will be very substantial. We hypothesize two extreme strategies in sticky glandular hairy carnivory plants (flypapers): (i) cheap and quickly re-establishable and (ii) costly, but waterproof and desiccation-resistant adhesives.
Because of a broad range of underwater applications in various fields, ranging from marine technology to medicine, current research interest in innovative reversible and reusable underwater adhesives increases [34–36]. Because traditional epoxy systems with strong reliable underwater bonding [31] are commonly known to be toxic, non-toxic adhesives of biological origin could offer a very promising alternative. That is why more detailed knowledge about the chemical composition of the R. gorgonias secretion might be potentially interesting as an inspiration for technical adhesives with underwater adhesive ability.
Acknowledgements
K. Keller (Augsburg, Germany) kindly provided plants and valuable information. The first author thanks M. Varenberg (Technion Israel Institute of Technology, Haifa, Israel) for his valuable comments and corrections and M. Voigt (Zwickau/Sa., Germany) for motivating discussions.
Funding statement
This study was partly supported by the COST Action TD0906.
References
- 1.Marloth R. 1910. Further observations on the biology of Roridula. Trans. R. Soc. South Afr. 2, 59–62. ( 10.1080/00359191009519362) [DOI] [Google Scholar]
- 2.Voigt D, Gorb SN. 2008. An insect trap as habitat: cohesion-failure mechanism prevents adhesion of Pameridea roridulae bugs to the sticky surface of the plant Roridula gorgonias. J. Exp. Biol. 211, 2647–2657. ( 10.1242/jeb.019273) [DOI] [PubMed] [Google Scholar]
- 3.Voigt D, Gorb EV, Gorb SN. 2009. Hierarchical organisation of the trap in the protocarnivorous plant Roridula gorgonias Roridulaceae. J. Exp. Biol. 212, 3184–3191. ( 10.1242/jeb.034280) [DOI] [PubMed] [Google Scholar]
- 4.Marloth R. 1903. Some recent observations on the biology of Roridula. Ann. Bot. 17, 151–158. [Google Scholar]
- 5.Ellis AG, Midgley JJ. 1996. A new plant-animal mutualism involving a plant with sticky leaves and a resisdent hemipteran insect. Oecologia 106, 478–481. ( 10.1007/BF00329705) [DOI] [PubMed] [Google Scholar]
- 6.Hartmeyer S. 1998. Carnivory in Byblis revisited II, the phenomenon of symbiosis on insect trapping plants. Carnivorous Plant Newsl. 27, 110–113. [Google Scholar]
- 7.Simoneit BRT, Medeiros PM, Wollenweber E. 2008. Triterpenoids as major components of the insect-trapping glue of Roridula species. Z. Naturforsch. 63c, 625–630. [DOI] [PubMed] [Google Scholar]
- 8.Marloth R. 1925. Flora of South Africa, vol. 2, Part I pp. 26–30. Cambridge, UK: University Press. [Google Scholar]
- 9.Lloyd FE. 1934. Is Roridula a carnivorous plant? Can. J. Res. 10, 780–786. ( 10.1139/cjr34-066) [DOI] [Google Scholar]
- 10.Bruce AN. 1907. On the distribution, structure, and function of the tentacles of Roridula. Notes R. Bot. Gard. Edinb. 17, 83–98. [Google Scholar]
- 11.Voigt D, Gorb SN. 2010. Desiccation resistance of adhesive secretion in the protocarnivorous plant Roridula gorgonias as an adaptation to periodically dry environment. Planta 232, 1511–1515. ( 10.1007/s00425-010-1270-2) [DOI] [PubMed] [Google Scholar]
- 12.Manning J. 2004. SASOL first field guide to Fynbos of Southern Africa. Cape Town, South Africa: Struik Publishers. [Google Scholar]
- 13.Lloyd FE. 1942. The carnivorous plants. New York, NY: The Ronald Press Company. [Google Scholar]
- 14.Barthlott W, Porembski S, Seine R, Theisen I. 2004. Karnivoren. Biologie und Kultur fleischfressender Pflanzen. Stuttgart, Germany: Ulmer Verlag. [Google Scholar]
- 15.Scherge M, Gorb SN. 2001. Biological micro- and nanotribology. Nature's solutions. Berlin, Germany: Springer. [Google Scholar]
- 16.Flammang P. 2006. Adhesive secretions in echinoderms: an overview. In Biological adhesives (eds Smith AM, Callow JA.), pp. 183–203. Berlin, Germany: Springer. [Google Scholar]
- 17.Waite JH. 1987. Nature's underwater adhesive specialist. Int. J. Adhes. Adhesives 7, 9–14. ( 10.1016/0143-7496(87)90048-0) [DOI] [Google Scholar]
- 18.Locke M. 1964. The structure and formation of the integument in insects. In The physiology of insecta (ed. Rockstein M.), pp. 123–213. New York, NY: Academic Press. [Google Scholar]
- 19.Rasche M. 1991. Gibt es einen Zusammenhang zwischen Benetzbarkeit und Haftung? Drauf & Dran 1991/1, 4–8 and 19–20.
- 20.Young T. 1805. An essay on the cohesion of fluids. Phil. Trans. R. Soc. Lond. 95, 65–87. ( 10.1098/rstl.1805.0005) [DOI] [Google Scholar]
- 21.Witten T, Pinus P. 2004. Structured fluids. Polymers, colloids, surfactants. New York, NY: Oxford University Press. [Google Scholar]
- 22.Tölpel A. 2007. Chemie und Physik der Milch. Naturstoff, Rohstoff, Lebensmittel, 2. Auflage, Hamburg: Behrs. [Google Scholar]
- 23.Bodamer GW, Kunin R. 1953. Behavior of ion exchange resins in solvents other than water. Ind. Eng. Chem. 45, 2577–2580. ( 10.1021/ie50527a057) [DOI] [Google Scholar]
- 24.Mantanis GI, Young RA, Rowell RM. 1994. Swelling of wood. Part 1. Swelling in water. Wood Sci. Technol. 28, 119–134. [Google Scholar]
- 25.Miller WS, Castagna CJ, Pieper AW. 2009. Understanding ion-exchange resins for water treatment systems. GE Water Process Technol. TP1050EN, 1–13. [Google Scholar]
- 26.Helfferich F. 1995. Ion exchange. New York, NY: Dover Publications. [Google Scholar]
- 27.DeVore DP, Gruebel RJ. 1978. Dityrosine in adhesive formed by the sea mussel Mytilus edulis. Biochim. Biophys. Res. Commun. 80, 993–999. ( 10.1016/0006-291X(78)91343-8) [DOI] [PubMed] [Google Scholar]
- 28.Yule AB, Walker G. 1984. The adhesion of the barnacle, Balanus balanoides, to slate surfaces. J. Mar. Biol. Assoc. UK 64, 147–156. ( 10.1017/S0025315400059695) [DOI] [Google Scholar]
- 29.Maruyama N, Etoh H, Sakata K, Ina K. 1991. Studies on phenoloxidase from Mytilus edulis associated with adhesion. Agric. Biol. Chem. 55, 2887–2889. ( 10.1271/bbb1961.55.2887) [DOI] [Google Scholar]
- 30.Dahlquist CA. 1969. Pressure-sensitive adhesives. In Treatise on adhesion and adhesives, vol. 2 (ed. Patrick RL.), pp. 219–260. New York, NY: Marcel Dekker. [Google Scholar]
- 31.Habenicht G. 2002. Kleben, Grundlagen, Technologien, Anwendung, 4th edn Berlin, Germany: Springer. [Google Scholar]
- 32.Schnepf E. 1969. Sekretion und Exkretion bei Pflanzen. Protoplasmatologia, Handbuch der Protoplasmaforschung, Bd. 8, Physiologie des Protoplasmas. New York, NY: Springer. [Google Scholar]
- 33.Rost K, Schauer R. 1977. Physical and chemical properties of the mucin secreted by Drosera capensis. Phytochemistry 16, 1365–1368. ( 10.1016/S0031-9422(00)88783-X) [DOI] [Google Scholar]
- 34.Varenberg M, Gorb SN. 2007. A beetle-inspired solution for underwater adhesion. J. R. Soc. Interface 5, 383–385. ( 10.1098/rsif.2007.1171) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Majumder A, Sharma A, Ghatak A. 2010. A bioinspired wet/dry microfluidic adhesive for aqueous environments. Langmuir 26, 521–525. ( 10.1021/la9021849) [DOI] [PubMed] [Google Scholar]
- 36.Dodou D, Breedveld P, de Winter JCF, Dankelman J, Leeuwen JL. 2010. Mechanisms of temporary adhesion in benthic animals. Biol. Rev. 86, 15–32. ( 10.1111/j.1469-185X.2010.00132.x) [DOI] [PubMed] [Google Scholar]