Figure 1.
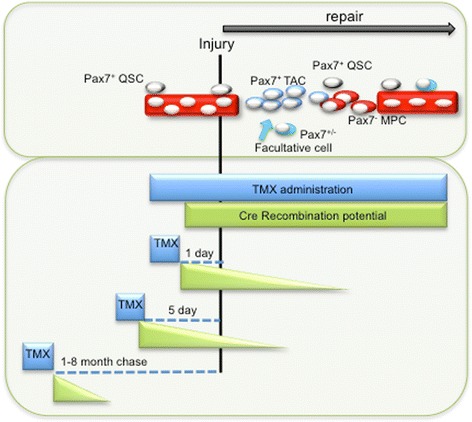
Strategies to target Pax7 cells using inducible Cre recombination and its implications for defining the cellular dynamics during muscle regeneration. Upper box: In response to injury, Pax7+ satellite cells exit from quiescence (QSC) (grey cells) to produce Pax7+ rapidly dividing transit amplifying progenitor cells (TAC, blue) that differentiate (myogenic progenitor cell, MPC), coupled to a decrease in Pax7 levels (-) (red) before forming new muscle. Proliferating Pax7+ SCs return to quiescence (grey cells) and reoccupy the niche. After injury, facultative cells (cyan) that express Pax7 transcript transiently (+/-) enter the muscle and contribute to the repair process. Lower box: Different TMX strategies (blue box) employed to target Pax7+ cells, before (followed by a chase: dashed line) and during injury. TMX half-life is estimated at around 10 days in vivo, therefore recombination of Pax7+ cells is possible following the TMX administration regimen (green box and triangle). If TMX is administered shortly before or during injury, Cre has the potential to recombine escaper Pax7+ QSCs as they proliferate (TAC). Other facultative cells that transiently express Pax7 transcript as part of the regeneration process and SCs that return back to quiescence can also be targeted. In all of these aforementioned scenarios, deletion of Pax7 gene if functionally important, will exacerbate the muscle phenotype, but at the expense of identifying the cell state that requires the gene. Targeting with TMX months prior to injury allows a definitive conclusion to be made against the QSC. This is critical since Pax7 levels differ across the QSC compartment and change dynamically during lineage progression.
