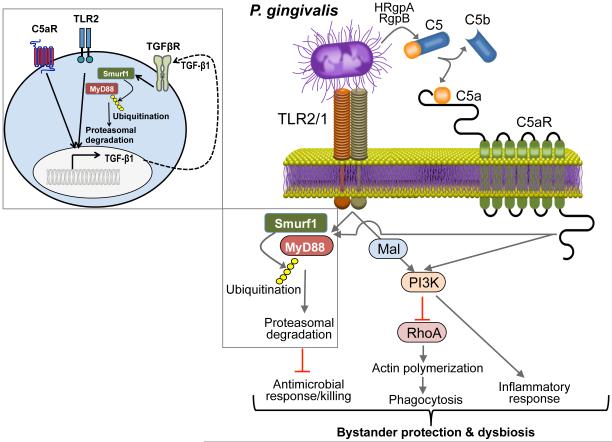
Figure 2. Porphyromonas gingivalis subversion of neutrophils leads to dysbiotic inflammation.
Porphyromonas gingivalis expresses ligands that activate the Toll-like receptor 2 (TLR1)–TLR2 complex and enzymes (HRgpA and RgpB gingipains) with C5 convertase-like activity that generate high local concentrations of C5a ligand. The organism can co-activate C5aR and TLR2 in neutrophils and the resulting crosstalk leads to ubiquitination and proteasomal degradation of the TLR2 adaptor MYD88, thereby inhibiting a host-protective antimicrobial response. This proteolytic event requires C5aR–TLR2-dependent release of transforming growth factor-β (TGF-β1), which mediates MYD88 ubiquitination via the E3 ubiquitin ligase Smurf1 (enlarged inset). Moreover, the C5aR–TLR2 crosstalk activates phosphoinositide 3-kinase (PI3K), which prevents phagocytosis through inhibition of RhoA GTPase and actin polymerization, while stimulating the production of inflammatory cytokines. In contrast to MyD88, another TLR2 adaptor, Mal, contributes to immune subversion by acting upstream of PI3K. These functionally integrated pathways, as manipulated by P. gingivalis, provide ‘bystander’ protection to otherwise susceptible bacterial species and promote polymicrobial dysbiotic inflammation in vivo. C5aR, complement C5a receptor; HRgpA, high molecular mass arginine-specific gingipain A; Mal, MyD88 adaptor-like; MyD88, myeloid differentiation primary response protein 88; RgpB, arginine-specific gingipain B; Smurf1, Smad ubiquitin regulatory factor 1. Reproduced with permission from REF.42.