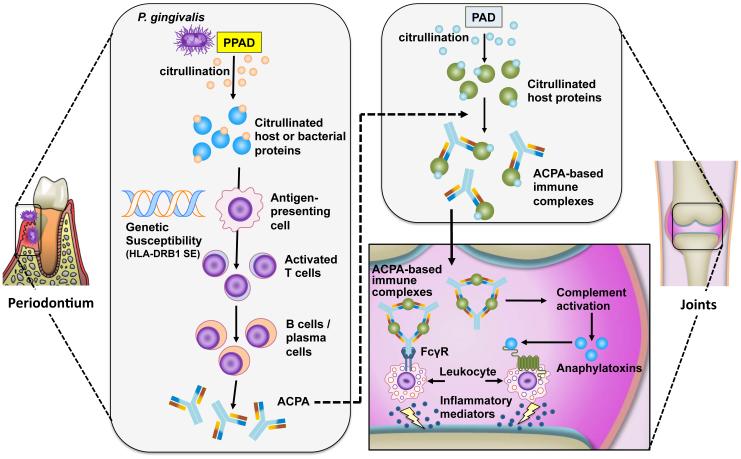
Figure 5. Porphyromonas gingivalis-mediated citrullination and induction of ACPA in rheumatoid arthritis.
P. gingivalis peptidylarginine deiminase (PPAD) citrullinates host-derived or bacterial proteins in the inflammatory environment of periodontitis. In susceptible individuals (carriers of HLA-DRB1 shared epitope (SE) alleles), distinct citrullinated peptides are presented in the context of HLA-DRB1 SE to activate T cells, which, in turn stimulate B-cell production of anti-citrullinated protein antibodies (ACPA). The induction of autoantibodies may be explained by mechanisms involving neoepitope formation or molecular mimicry. Citrullination of host proteins, such as α-enolase, fibrinogen and collagen type II, by human peptidylarginine deiminase (PAD) enzymes can occur in injured or inflamed joints. ACPA bind citrullinated proteins and form immune complexes that can mediate local synovial inflammation by activating complement or Fcγ receptors (FcγR).