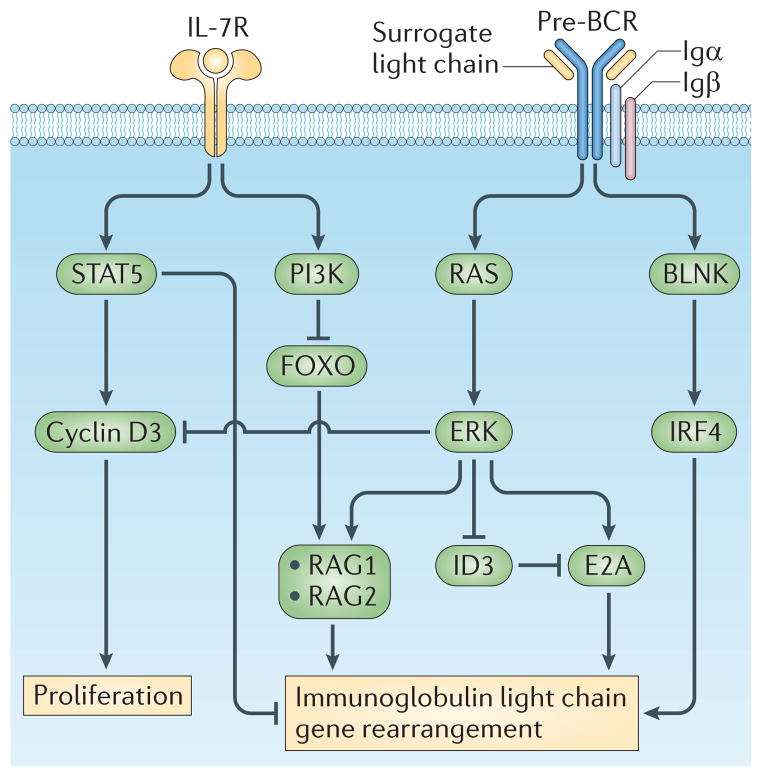
Figure 2. The IL-7R and pre-BCR coordinate proliferation with Igκ gene recombination in B lineage cells.
Downstream of each receptor, distinct signalling pathways have specific functions in proliferation and recombination. Interleukin-7 receptor (IL-7R)-mediated signal transducer and activator of transcription 5 (STAT5) activation induces transcription of cyclin D3, which promotes proliferation. In addition, STAT5 directly represses Igκ gene accessibility and recombination. The IL-7R also activates phosphoinositide 3-kinase (PI3K), which represses forkhead box protein O1 (FOXO1), an obligate inducer of recombination-activating gene 1 (RAG1) and RAG2 gene transcription. By contrast, the pre-B cell receptor (pre-BCR) is coupled to the RAS–extracellular signal-regulated kinase (ERK) signalling pathway, which represses cyclin D3 and inhibitor of DNA binding 3 (ID3) while inducing E2A. This has the effect of inhibiting proliferation and increasing levels of free nuclear E2A. E2A with interferon regulatory factor 4 (IRF4), downstream of B cell linker protein (BLNK), coordinately enhance Igκ gene accessibility. Preferential coupling of the PI3K pathway to the IL-7R, and not the pre-BCR, ensures that each receptor has opposing and antagonistic functions. The IL-7R induces proliferation and represses Igκ gene recombination while the pre-BCR represses proliferation and induces recombination.