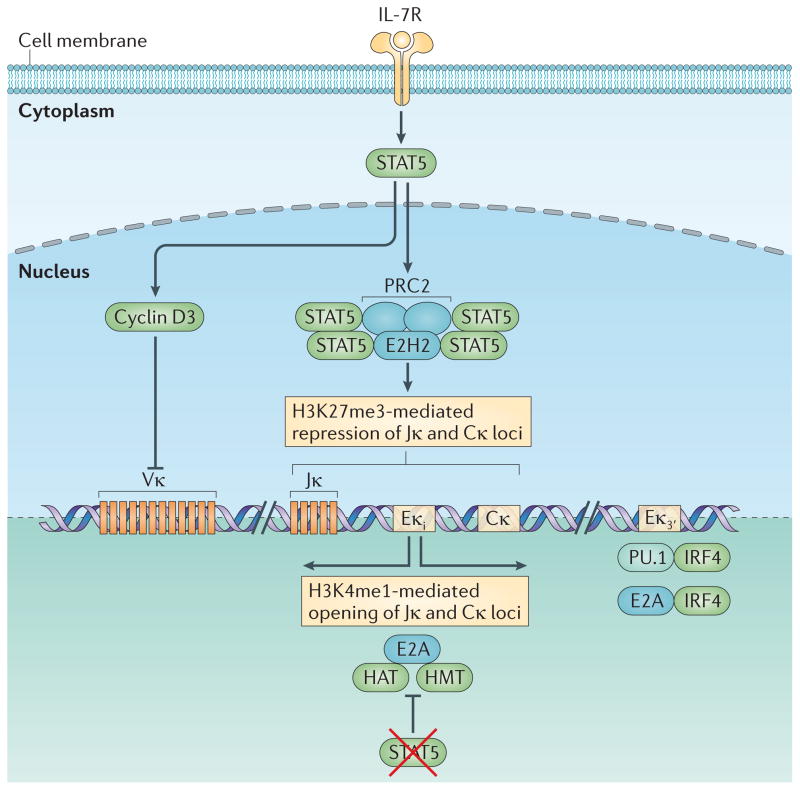
Figure 4. Regulation of Igκ locus accessibility.
Stimulation of the interleukin-7 receptor (IL-7R) induces the activation of signal transducer and activator of transcription 5 (STAT5). Within the Igκ intronic enhancer (Eκi), phosphorylated STAT5 binds as a tetramer, instead of as a dimer; this enables it to recruit Polycomb repressive complex 2 (PRC2), which contains the histone methyltransferase enhancer of zeste homologue 2 (EZH2). EZH2 marks the region, containing Jκ and Cκ, with histone H3 lysine 27 trimethylation (H3K27me3), thereby conferring local epigenetic repression. STAT5 also induces the expression of cyclin D3, which potently represses Vκ accessibility. The mechanism by which it does so is unclear and neither seems to require direct chromatin binding nor involve changes in post-translational histone modifications. Induction of Igκ requires escape from IL-7R signalling, the consequent loss of activated STAT5 and the downregulation of the STAT5 target cyclin D3. However, loss of IL-7R signalling is not sufficient for opening of the Igκ locus. Pre-B cell receptor (pre-BCR)-mediated extracellular signal-regulated kinase (ERK) activation and downstream induction of free nuclear E2A is required. With the loss of phospho-STAT5 binding, E2A binds to the Eκi, where it recruits histone acetyltransferases (HATs) and histone methyltransferases (HMTs) that provide histone marks that lead to the opening of Jκ and Cκ to recombination. E2A also binds to the Eκ3′, where it cooperates with pre-BCR-induced interferon-regulatory factor 4 (IRF4) to further enhance Igκ accessibility. IRF4 also binds in a cooperative manner with the transcription factor PU.1 to a distinct composite regulatory sequence in Eκ3′ to regulate its activation.