Abstract
Tuberculosis (TB) is a major global public health problem with an estimated one-third of the world’s population infected with Mycobacterium tuberculosis, thus necessitating fast and accurate diagnosis of TB. However, the diagnosis of latent infection remains difficult due to the lack of a simple, reliable test for M. tuberculosis infection. The objective of the current study was to generate and characterize a novel monoclonal antibody (mAb) that specifically target the early secretory antigenic target 6 (ESAT-6) protein for tuberculosis (TB) immunological diagnosis. The BALB/c mice were immunized by a peptide with 13 amino acids (amino acid residues 24 to 36) of ESAT-6 protein. Stable hybridomas cell lines were established and mAb was identified by Western Blot, immunoprecipitation (IP) and Enzyme linked immunosorbent assay (ELISA). In addition, this mouse mAb was used to coat plates, and biotin-labelled polyclonal antibodies were used to detect the antigens. Finally, the antibody was verified in 280 patient sputum culture supernatants and pleural effusion aspirates. The positive detection rate of the generated ESAT-6 mAb was 91% in the clinical diagnosis of tuberculosis pleural effusion, and this mAb had a sensitivity of 92.4% and a specificity of 100% in all M. tuberculosis cases. Our data indicated that the mAb generated in this study can potentially serve as a tool in the laboratory diagnosis of TB.
Keywords: Anti-ESAT-6, monoclonal antibody, immunological detection, Mycobacterium tuberculosis
Introduction
Tuberculosis (TB) is a major global public health problem, and it is estimated that one-third of the world’s population is infected with Mycobacterium tuberculosis (M. tuberculosis) [1]. The major cause of TB is M. tuberculosis which is an intracellular pathogen. Fast and accurate diagnosis of TB is an important element in controlling this disease. However, the diagnosis of latent infection remains difficult due to the lack of a simple, reliable test for M. tuberculosis infection. Currently, M. tuberculosis infection can be diagnosed with the help of the tuberculin skin test (TST) using purified protein derivative (PPD), but this conventional method has known limitations in accuracy and reliability. Furthermore, interpretation of serial TST results is complicated by non-specific variation and because of its intradermal application, by potential boosting from precedent tests [2]. Conversion of the PPD reactivity from negative to positive challenges the clinician [3].
Genome of M. tuberculosis H37Rv consists of 16 regions of differences (RD) ranging from 2 to 12.7 kb, which are deleted in most BCG variants and members of TBC [4]. Nine of the RDs (containing 61 ORFs) are absent from all the BCG strains as well as all virulent M. bovis strains [4]. One key deletion, RD1, was missing from all the BCG strains but present in all other M. tuberculosis complex members including M. bovis [4]. The RD regions are largely responsible for the heterogeneity in their epidemiological and clinical behavior. The RD1 locus plays a key role in the virulence of M. tuberculosis [5]. Among the major antigens of RD1 locus, comparative genomic studies have revealed two genes, early secretory antigenic target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) exclusively present in several pathogenic mycobacterial species, including M. tuberculosis and non-tuberculosis species [6]. ESAT-6 is the best characterized protein within the RD1 region. It has been recognized that ESAT-6 can induce a strong T-cell-mediated immune response both in vitro and in vivo [7]. Also, it has been proposed as a tool for diagnosis of M. tuberculosis infection and frequently used in enzyme-linked immunospot assay (ELISPOT) [8,9].
Based on these findings, we proposed that ESAT-6 could be acted as a target for developing novel mAbs in TB immunological detection. Specific mAbs against M. tuberculosis may prove to be ideal reagents for diagnosing TB infection. In the present study, we generated and characterized a new mAb that specifically anti ESAT-6 protein. This development has great utility for immunoblotting, IP and ELISA. Moreover, the isotype of this mAb was an IgG2b with a kappa chain. Clinical validation of this mAb showed that it has highly detection and sensitivity rates of ESAT-6 in TB cases. Thus, this mAb will provide a powerful tool for the laboratory diagnosis of TB infection.
Materials and methods
Bio-synthesis of the target peptide
The target peptide consisted of 13 amino acids (aa), ranging from 24 to 36 residues of ESAT-6 protein (aa sequence: SIHSLLDEGKQSL), was synthesized by a conventional solid-state reaction technique (Applied Biosystems, Inc., CA, USA), based on the widely used Fmoc strategy (optimized for antigenicity and specificity), using the Wang resin as the solid support [10]. After synthesis, the peptides were cleaved from the resin and purified in High Performance Liquid Chromatography (HPLC), and the mass spectrometry (MS) was carried out to evaluate the quality of the synthesized peptides (purity > 95%).
Immunization of mice
BALB/c mice were obtained from Shanghai Experimental Animal Centre of the Chinese Academy of Sciences (Shanghai, China). Animal experiments were performed in accordance with the guidelines of the Chinese Council on Animal Care and approved by Hangzhou First People’s Hospital Committees on Animal Experimentation. The female mice, six weeks old, receiving the regular injections of 0.2 ml ESAT-6 antigen were immunized one time with a two-week interval, repeated four times. Six weeks after enhanced immunization, the mice were killed and the lymphoblasts were obtained from the spleen for the next fusion stage.
Generation of anti-ESAT-6 mAb
Mouse myeloma Sp2/0 cells were purchased from American Type Culture Collection (Manassas, VA, USA), and was grown in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine serum (FBS). One day before fusion, the peritoneal macrophages from the normal BALB/c mice were used as feeder layer cells. Spleen cells from immunized animals were fused with Sp2/0 myeloma cells according to the protocol that had been previously described [11]. More than 100 independent hybridomas were obtained from two fusions. Positive hybridomas clones were selected by coating-antigen ELISA. To obtain the stable mAb-expression hybridomas, a limiting dilution was performed. The pristine-primed BALB/c mice were injected intraperitoneally with 5×106 hybridoma cells per mouse to acquire abundant mAbs. Ten days later, the ascitic fluid was collected and mAbs were purified using a Protein A/G affinity column, according to the manufacturer’s instructions [12]. Antibody isotypes were identified using a Mouse Monoclonal Antibody Isotyping Test Kit (AbD Serotec), according to the manufacturer’s instructions. After purification, the mAb was biotin-labelled using biotin labelling reagent according to the manufacturer’s protocol (Thermo Scientific, Huntsville, AL).
Characterization of anti-ESAT-6 mAb
Co-Immunoprecipitation (Co-IP) was performed to characterize the anti-ESAT-6 mAb. Individual samples (1 μg recombinant protein/sample) were subjected to immunoprecipitation by ESAT-6 mAB (1 μg/sample) that were coupled to protein G Sepharose beads. The immunoprecipitates were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted with specific goat anti-ESAT-6 antibody followed by detection through an enhanced chemiluminescence reaction. Results were recorded and analyzed by VersaDoc MP5000 Imaging System (Bio-Rad Laboratory, Hercules, CA, USA).
Clinical validation
Thirty-eight sputum culture supernatants positive for M. tuberculosis but negative for other mycobacteria, twenty sputum culture supernatants negative for M. tuberculosis but positive for other mycobacteria and twenty-two samples which were diagnosed as tuberculous pleural effusion were obtained from Red Cross Hospital of Hangzhou, China. All patients were diagnosed according to the American Thoracic Society guidelines [13] and Small et al. [14]. Twenty-four sputum culture supernatants negative for M. tuberculosis but positive for other mycobacteria and twenty clinically diagnosed non-tuberculous pleural effusion samples were obtained from patients as health controls from Hangzhou First People’s Hospital. Informed written consent was obtained from all participants. The mAb against ESAT-6 in pleural fluid and culture samples were tested using the ELISA method.
Results
Isotype of mouse mAb against ESAT-6
The hybridomas that reacted selectively with ESAT-6 in all assays were further evaluated. The isotype of this mAb, determined by a Mouse Monoclonal Antibody Isotyping Test Kit (AbD Serotec), was an IgG2b with a kappa chain (Figure 1).
Figure 1.
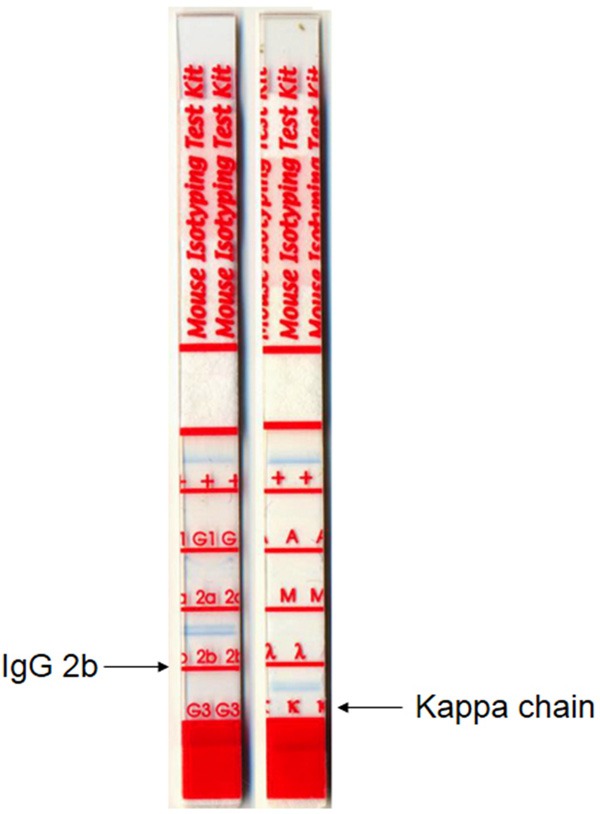
The mAb isotype, determined by a mouse monoclonal isotyping kit, was an IgG2b with a Kappa chain.
Characterization of mAb anti-ESAT-6
We further used co-immunoprecipitation and ELISA approaches to characterize the newly generated anti-ESAT-6 mAb. Figure 2 indicates the mAb can specifically interact and identify ESAT-6 and CFP-10-ESAT-6 fusion proteins [15], which also revealed that this mAb can specifically recognize ESAT-6 in different M. tuberculosis. We also generated a standard curve for calculating the expression of ESAT-6 from each sample set that was assayed (Figure 3).
Figure 2.
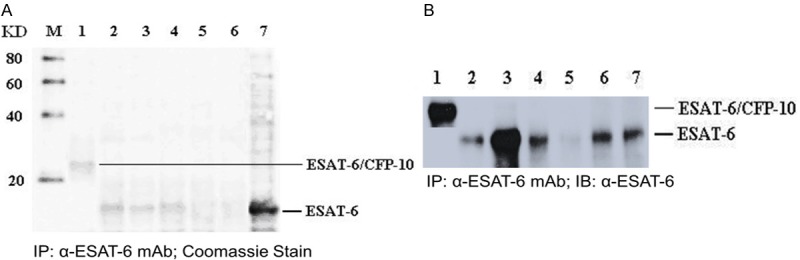
A. IP-Coomassie Stain. B. IP-western blot detection of the cultivation of the mAb raised against the ESAT-6 protein in hybridoma cells. M = Marker, lane 1 = ESAT-6/CFP-10 fusion protein, lane 2 = recombined ESAT-6 protein, lane 3 = Mycobacterium tuberculosis culture supernatant, lane 4 = Tuberculous pleural effusion, lane 5 = Bird intracellular mycobacterial lysis protein, lane 6 = Kansas mycobacterial lysis protein, lane 7 = Lysis protein of Mycobacterium tuberculosis.
Figure 3.

Standard curve of ESAT-6 generated by ELISA. Different concentrations of ESAT-6 protein were used as standards, and the biotinylated polyclonal antibody was used as a detection antibody. Results were visualized by adding streptavidin-HRP and TMB substrates and the optical density of the plates was read at 450 nm using an ELISA reader.
ELISA method based on mAb in the clinical detection of M. tuberculosis
The sandwich ELISA that we previously developed [15] showed that the ESAT-6 mAb test had a sensitivity of 92.0% and a specificity of 100% in all M. tuberculosis cases. As summarized in Table 1, the positive detection rate of ESAT-6 was 92% (46/50) in the clinical diagnosis of tuberculous pleural effusion.
Table 1.
Results of ELISA kit development that was tested on clinical samples used in this study
| Tuberculosis status | Subjects n | Positive for ESAT-6 |
|---|---|---|
| Sputum culture supernatants positive for Mycobacterium tuberculosis | 65 | 65 |
| Sputum culture supernatants negative for M. tuberculosis but positive for other mycobacteria | 65 | 0 |
| Clinical diagnosis of tuberculous pleural effusion (sputum culture-positive for M. tuberculosis) | 50 | 46 |
| Clinical diagnosis of tuberculous pleural effusion (sputum culture-negative for M. tuberculosis) | 50 | 0 |
| Clinical diagnosis of non-tuberculous pleural effusion | 50 | 0 |
ELISA, enzyme linked immunosorbent assay; ESAT-6, early-secreted antigenic target 6.
Discussion
Currently, the time-consuming M. tuberculosis culture (4-8 weeks) remains the gold standard in the diagnosis of active TB disease. Late diagnosis and treatment of M. tuberculosis increase the risk of disease dissemination and decreases the survival of some subgroups of patients [16,17]. Therefore, early diagnosis and treatment of TB patients are crucial for the control of TB spread. Various studies have been undertaken to develop serodiagnostic test using proteins of M. tuberculosis which are known to be immunodominant and early markers for TB [18].
These years, comparative genomic studies using subtractive DNA hybridization [19] and DNA microarray [4] have identified several M. tuberculosis-specific genomic regions of difference (RD), designated RD1 to RD16, which are absent in the vaccine strains of M. bovis bacillus Calmette-Guérin (BCG), indicating that the RD-encoded recombinant proteins may be useful for improving the sensitivity and specificity of serodiagnosis TB. RD1 presented in all virulent members of M. tuberculosis and M. bovis [20]. Several antigenic proteins of M. tuberculosis encoded by genes located in RD1 have potential for specific diagnosis of TB. The best-studied RD1 proteins are ESAT-6 and CFP-10, both of which elicit strong T-cell and B-cell responses in experimental animals and humans [21,22]. ESAT-6 and CFP-10 are dominant antigens that induce the secretin of IFN-γ specific to tuberculous mycobacteria and are absent from many environmental non-tuberculous mycobacteria [8]. Previous studies showed that the recombinant ESAT-6/CFP-10 fusion protein is useful for detection tuberculous cattle in herbs with pre-existing sensitization to M. avium or M. avium subsp. paratuberculosis.
In the present study, we developed a novel mAb against ESAT-6 by employing a monoclonal antibody technique. Considering ESAT-6 is a relatively small antigen, we therefore generated a 13 lengthened peptide using a solid-state reaction approach and immunized the BALB/c mice to gain abundant antibody. We further generated the hybridomas using a conventional method. We also immunized the mice of the hybridomas to get enough mAb and the specificity of this mAb was evaluated by ELISA, IP and Western Blot.
Our results suggested that this mAb was reactive in both IP and Western Blot, and clinical validation of this mAb showed that it could be used as a potential tool to detect TB. Compared to a previous study of generation the novel mAbs to ESAT-6 and CFP-10 antigens [14], the mAb generated in this study has a positive detection rate of 92.0% (46/50) and a negative detection rate of 100%. The mAb has a much higher sensitivity than the ESAT-6 and CFP-10 in the ELISA test for TB detection. The tuberculin skin test (TST) using purified protein derivative (PPD) is most commonly used test for TB, but this conventional method detection is of limited use in China because of the high BCG vaccination rates and ensuing false positives with the TST. In conclusion, we prepared and identified a novel mAb that could be used in TB diagnosis.
Acknowledgements
This work was supported by the grant from Hangzhou Bureau of Science and Technology (No. 20100633B01) and the grants from the National Twelfth Five-year Plan Key Program of Major Infectious Diseases (Grant No. 2012ZX10001-003) of China.
Disclosure of conflict of interest
None.
References
- 1.World Health Organization. Global tuberculosis control: surveillance, planning, financing: WHO report. Geneva, Switzerland: World Health Organization; 2007. [Google Scholar]
- 2.Pai M, Dheda K, Cunningham J, Scano F, O’Brien R. T-cell assays for the diagnosis of latent tuberculosis infection: moving the research agenda forward. Lancet Infect Dis. 2007;6:428–38. doi: 10.1016/S1473-3099(07)70086-5. [DOI] [PubMed] [Google Scholar]
- 3.Fine PE, Bruce J, Ponnighaus JM, Nkhosa P, Harawa A, Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–75. [PubMed] [Google Scholar]
- 4.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 5.van Ingen J, de Zwaan R, Dekhuijzen R, Boeree M, van Soolingen D. Region of difference 1 in nontuberculous Mycobacterium species adds a phylogenetic and taxonomical character. J Bacteriol. 2009;191:5865–7. doi: 10.1128/JB.00683-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganguly N, Giang PH, Gupta C, Basu SK, Siddiqui I, Salunke DM, Sharma P. Mycobacterium tuberculosis secretory proteins CFP-10, ESAT-6 and the CFP10:ESAT6 complex inhibit lipopolysaccharide-induced NF-kappaB transactivation by downregulation of reactive oxidative species (ROS) production. Immunol Cell Biol. 2008;86:98–106. doi: 10.1038/sj.icb.7100117. [DOI] [PubMed] [Google Scholar]
- 7.Guinn KM, Hickey MJ, Mathur SK, Zakel KL, Grotzke JE, Lewinsohn DM, Smith S, Sherman DR. Individual RD1-region genes are required for export of ESAT-6/CFP-10 and for virulence of Mycobacterium tuberculosis. Mol Microbiol. 2004;51:359–70. doi: 10.1046/j.1365-2958.2003.03844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marei A, Ghaemmaghami A, Renshaw P, Wiselka M, Barer M, Carr M, Ziegler-Heitbrock L. Superior T cell activation by ESAT-6 as compared with the ESAT-6-CFP-10 complex. Int Immunol. 2005;17:1439–46. doi: 10.1093/intimm/dxh322. [DOI] [PubMed] [Google Scholar]
- 9.Wang JY, Chou CH, Lee LN, Hsu HL, Jan IS, Hsueh PR, Yang PC, Luh KT. Diagnosis of tuberculosis by an enzyme-linked immunospot assay for interferon-gamma. Emerg Infect Dis. 2007;13:553–8. doi: 10.3201/eid1304.051195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart JM, Young JD. Solid Phase Peptide Synthesis. 2nd edition. Pierce; 1979. pp. 105–7. [Google Scholar]
- 11.Yao HP, Luo YL, Feng L, Cheng LF, Lu Y, Li W, Wang MH. Agonistic monoclonal antibodies potentiate tumorigenic and invasive activities of splicing variant of the RON receptor tyrosine kinase. Cancer Biol Ther. 2006;5:1179–86. doi: 10.4161/cbt.5.9.3073. [DOI] [PubMed] [Google Scholar]
- 12.Wang B, Liu Y, Shan Y, Yao Z, Liu X, Su R, Sun Q, Cong Y, Li J. Generation and characterization of novel human IRAS monoclonal antibodies. J Biomed Biotechnol. 2009;2009:973754. doi: 10.1155/2009/973754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This Official Statement of the American Thoracic Society and the Centers for Disease Control and Prevention was Adopted by the ATS Board of Directors, July 1999. This Statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–95. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 14.Small PM, Pai M. Tuberculosis diagnosis--time for a game change. N Engl J Med. 2010;363:1070–1. doi: 10.1056/NEJMe1008496. [DOI] [PubMed] [Google Scholar]
- 15.Feng TT, Shou CM, Shen L, Qian Y, Wu ZG, Fan J, Zhang YZ, Tang YW, Wu NP, Lu HZ, Yao HP. Novel monoclonal antibodies to ESAT-6 and CFP-10 antigens for ELISA-based diagnosis of pleural tuberculosis. Int J Tuberc Lung Dis. 2011;6:804–10. doi: 10.5588/ijtld.10.0393. [DOI] [PubMed] [Google Scholar]
- 16.Pablos-Méndez A, Sterling TR, Frieden TR. The relationship between delayed or incomplete treatment and all-cause mortality in patients with tuberculosis. JAMA. 1996;276:1223–8. doi: 10.1001/jama.1996.03540150025026. [DOI] [PubMed] [Google Scholar]
- 17.Alwood K, Keruly J, Moore-Rice K, Stanton DL, Chaulk CP, Chaisson RE. Effectiveness of supervised, intermittent therapy for tuberculosis in HIV-infected patients. AIDS. 1994;8:1103–8. doi: 10.1097/00002030-199408000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Målen H, Søfteland T, Wiker HG. Antigen analysis of Mycobacterium tuberculosis H37Rv culture filtrate proteins. Scand J Immunol. 2008;67:245–52. doi: 10.1111/j.1365-3083.2007.02064.x. [DOI] [PubMed] [Google Scholar]
- 19.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;5:1274–82. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hart PD, Sutherland I. BCG and vole bacillus vaccines in the prevention of tuberculosis in adolescence and early adult life. Br Med J. 1977;6082:293–5. doi: 10.1136/bmj.2.6082.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arend SM, Geluk A, van Meijgaarden KE, van Dissel JT, Theisen M, Andersen P, Ottenhoff TH. Antigenic equivalence of human T-cell responses to Mycobacteriumtuberculosis-specific RD1-encoded protein antigens ESAT-6 and culture filtrate protein 10 and to mixtures of synthetic peptides. Infect Immun. 2000;6:3314–21. doi: 10.1128/iai.68.6.3314-3321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brusasca PN, Colangeli R, Lyashchenko KP, Zhao X, Vogelstein M, Spencer JS, McMurray DN, Gennaro ML. Immunological characterization of antigens encoded by the RD1 region of the Mycobacterium tuberculosis genome. Scand J Immunol. 2001;54:448–52. doi: 10.1046/j.1365-3083.2001.00975.x. [DOI] [PubMed] [Google Scholar]


