Abstract
Objectives: This study is to determine if two adipocytokines, adiponectin and visfatin, can be used as diagnosis markers for metabolic syndrome (MS) in Uygur population. Methods: Sixty-two MS patients and 41 control individuals with normal body weights were enrolled in this study. Abdominal subcutaneous and omental adipose tissues were collected for determination of biochemical indices. The adipokines serum levels were determined by enzyme-linked immunosorbent assay (ELISA). Blood were collected from the MS patients and the control individuals and extracted proteins and RNAs subjected to western blot analysis and real-time PCR to determine adiponectin and visfatin expression, respectively. Results: ELISA indicated that the serum adiponectin in the MS group was decreased (0.59 ± 0.21 versus 0.49 ± 0.18) in comparison with the control group (P < 0.05). But the serum visfatin in the MS group were increased (1.07 ± 0.41 versus 1.25 ± 0.32) when compared with the control group (P < 0.05). The western blot revealed decreased adiponectin and increased visfatin expression in the MS patients when compared with the normal controls. Further real-time RT-PCR analysis showed that the adiponectin and visfatin expression are altered via a transcriptional mechanism. Conclusions: Adiponectin and visfatin might be used as diagnosis markers of MS in Uygur population.
Keywords: Metabolic syndrome, adipocytokine, adiponectin, visfatin
Introduction
Metabolic syndrome (MS) has become a severe public health problem in the world and affects people across various ethnicities in various countries [1]. MS is a group of medical abnormalities, such as obesity, insulin resistance, glucose intolerance, hyperlipidemia and hypertension. It has been reported that MS contributes to development of cardiovascular disease and chronic kidney disease [2-5]. Some studies have shown that prevalence of MS increases with age [6]. The adipose tissue or body fat, which is closely related to MS, is a type of loose connective tissue composed mostly of adipocytes that are derived from preadipocytes [7]. Adipose tissue is a major endocrine organ, since it secretes hormones including estrogen, leptin, resistin and a cytokine TNF-α. Furthermore, adipose tissues may affect other organ systems of the body, thus contributing to MS.
Expression of some genes has been reported to be related to MS. For example, suppressor of cytokine signaling 3 (SOCS3) is found to act as an inhibitor for a group of cytokine signals and inhibits functions of MS-related hormones such as leptin and the downstream steps in the insulin signaling pathway [8,9]. In addition, some infectious factors may be related to MS. It has been found that some viral infection, such as hepatitis B virus, hepatitis C virus and hepatitis D virus, may be related to MS in liver transplantation patients [10,11]. We have recently found that a big portion of the long-term obese Uygur residents in Xinjiang region, China, are naturally infected with Adenovirus type 36.
Some peptides, such as adiponectin [12] and visfatin [13], are referred to as adipokines, which can also be put into the list of adipose-derived hormones. In this study, Uygur MS patients and the Uygur individuals with normal body weights were enrolled. The expression of adiponectin and visfatin in these Uygur MS patients and the Uygur individuals with normal body weights has been investigated.
Materials and methods
Patients
The MS diagnosis in China was performed according to the world consensus definition. The MS patients must fit with the standard for obesity (i.e. Male waistline ≥ 90 cm, Female waistline ≥ 80 cm) and two of the following factors: (i) triglyceride (TG) levels was increased (> 1.7 mmol/L); (ii) high density lipoprotein cholesterol (HDL-C) levels was decreased (Male, < 1.03 mmol/L; Female, < 1.29 mmol/L); (iii) Blood pressure was increased (systolic blood pressure (SBP) ≥ 130 mmHg and diastolic blood pressure (DBP) ≥ 85 mmHg); and (iv) Fasting glucose was increased (≥ 5.6 mmol/L). Sixty-two MS patients and 41 control individuals with normal body weights were enrolled in this study. Written and informed consent were obtained from every patient and the study was approved by the ethics review board of People’s Hospital of Xinjiang Uygur Autonomous Region.
Specimen collection and blood tests
Abdominal subcutaneous and omental adipose tissues (500 mg) were collected from every MS patients and the normal control individuals. Venous blood (5 mL) was collected. Cholesterol oxidase test was performed to test the total cholesterol (TC). Polyethylene sulfate test was performed to test low density lipoprotein cholesterol (LDL-C) levels. Enzymatic assay test was performed to determine the values of TG and HDL-C. Glucose oxidase-peroxidase-coupled method was used to measure the fasting blood glucose (FBG) levels.
Quantitative reverse transcription-PCR (RT-PCR)
The RNAs were extracted from adipose tissues by Trizol (Invitrogen Corporation, USA). The RT-PCR experiments were repeatedly performed at least for 3 times. RNA was reverse-transcribed using random primers with a reverse transcription II system (Promega, Madison, WI, USA). The levels of mRNAs were determined with an ABI Prism System (Applied Biosystems, USA). Primers used in this study were given in Table 1. In the PCR, enzyme-negative and template-negative samples were used as negative controls. Amplification of adiponectin and visfatin cDNAs and the endogenous β-actin cDNA were measured by changes in FAM and VIC fluorescence, respectively, using the ABI 7500 software. The relative levels of transcripts were normalized to the mRNA levels of β-actin in the same sample.
Table 1.
Primers used in this study
| Primers | Sequences |
|---|---|
| β-actin_F | 5’-GCTATCCAGGCTGTGCTATC |
| β-actin_R | 5’-CAGCACTGTGTTGGCGTACA |
| Adiponectin_F | 5’-GTCCTAAGGGAGACATCGGT |
| Adiponectin_R | 5’-TGCAGTGGAATTTACCAGTGG |
| Visfatin_F | 5’-CTGATTCTGGAAACCCTC |
| Visfatin_R | 5’-CTGGCGTCCTATGTAAAG |
Enzyme-linked immunosorbent assay (ELISA)
The serum cytokine concentrations in the two groups were measured by using ELISA kit purchased from Beijing Aidi Bo biological company (Beijing, China). The optical density (OD) values were determined by a microplate reader at a wave length of 450 nm. Standard curve was drawn to calculate the concentration of each sample.
Western blot analysis
Total proteins were harvested from blood collected from the MS patients and the control individuals and then separated on SDS/PAGE gels, and then determined by immunoblot analyses. Primary antibodies against adiponectin (30 kDa), visfatin (about 52 kDa) and β-actin were purchased from Santa Cruz company, USA (adiponectin, sc-136131, 1:200; anti-visfatin, sc-130058, 1:200; anti-actin, sc-130301, 1:10,000). The secondary antibodies were purchased from Pierce Biotechnology. The signals were detected with the ECL detection system (Pierce Biotechnology, USA). The experiments were repeated for more than 3 times.
Statistical analyses
The experimental data were shown as mean ± SD. A statistical software (SPSS10.0, Chicago, USA) was used for performance of the Student’s t Test. Data with P < 0.05 was considered as significant.
Results
MS patients have obvious clinical characteristics of MS
As given in Table 2, the ages between these two groups were similar. BMI, WC, HC, DBP, SBP, FPG, TC, and TG in the MS group were significantly increased when compared with the control group (P < 0.05). However, HDL-C and LDL-C values in the MS group were significantly decreased in comparison with the data in the control group (P < 0.05). The above results suggested that MS patients have obvious clinical characteristics of MS.
Table 2.
Information of MS patients and the normal control individuals (mean + SD)
| Parameters | Normal control group | MS group |
|---|---|---|
| Age | 53 ± 11 | 54 ± 8 |
| BMI (kg/m2) | 23.9 ± 4.1 | 28.6 ± 4.2* |
| WC (cm) | 86.0 ± 10.8 | 99.5 ± 8.5* |
| HC (cm) | 98.3 ± 8.9 | 106.3 ± 8.3* |
| DBP (mmHg) | 73.6 ± 9.4 | 81.2 ± 17.9* |
| SBP (mmHg) | 115.6 ± 10.2 | 128.0 ± 23.8* |
| FPG (mmol/L) | 5.17 ± 1.24 | 6.45 ± 1.99* |
| TC (mmol/L) | 3.91 ± 0.80 | 4.48 ± 0.68* |
| TG (mmol/L) | 1.21 ± 0.51 | 2.07 ± 1.02* |
| HDL-C (mmol/L) | 1.36 ± 0.58 | 1.27 ± 0.61* |
| LDL-C (mmol/L) | 2.14 ± 0.67 | 1.26 ± 0.61* |
p < 0.05, compared with the normal control.
BMI, body mass index; WC, waistline; HC, hipline; DBP, diastolic blood pressure; SBP, systolic blood pressure; FPG, fasting plasma glucose; TC, total cholesterol; TG, triglerid; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Serum adipokines were significantly altered in the MS group
As given in Table 3, serum adiponectin in the MS group was decreased (0.59 ± 0.21 versus 0.49 ± 0.18) in comparison with the control group (P < 0.05). But serum visfatin in the MS group were increased (1.07 ± 0.41 versus 1.25 ± 0.32) in comparison with the control group (P < 0.05). In addition, the progranulin and omental adiponectin levels were also significantly altered (P < 0.05). These results suggest that expressions of adiponectin and visfatin may be used as diagnosis markers of MS in Uygur population.
Table 3.
Levels of adipocytokines in the MS patients and the normal control individuals (mean + SD)
| Parameters | Normal control group | MS group |
|---|---|---|
| Chemerin (ng/ml) | 28.27 ± 17.85 | 26.97 ± 16.65 |
| Serum adiponectin (ng/ml) | 0.59 ± 0.21 | 0.49 ± 0.18* |
| Serum visfatin (ng/ml) | 1.07 ± 0.41 | 1.25 ± 0.32* |
| Leptin (pg/ml) | 206.48 ± 106.20 | 212.70 ± 72.95 |
| Progranuli (pg/ml) | 443.61 ± 115.89 | 300 ± 158.64* |
| Chemerin (omental) | 1.21 ± 0.90 | 0.92 ± 0.80 |
| Chemerin (subcutaneous) | 0.33 ± 0.15 | 0.30 ± 0.14 |
| Adiponectin (omental) | 0.25 ± 0.06 | 0.22 ± 0.04* |
| Adiponectin (subcutaneous) | 0.25 ± 0.06 | 0.23 ± 0.05 |
| Visfatin (omental) | 0.57 ± 0.10 | 0.54 ± 0.10 |
| Visfatin (subcutaneous) | 0.60 ± 0.11 | 0.60 ± 0.13 |
| Leptin (omental) | 0.23 ± 0.17 | 0.21 ± 0.10 |
| Leptin (subcutaneous) | 0.22 ± 0.11 | 0.22 ± 0.08 |
| Progranulin (omental) | 1.00 ± 0.002 | 1.00 ± 0.003 |
| Progranulin (subcutaneous) | 1.00 ± 0.002 | 1.00 ± 0.002 |
p < 0.05, compared with the normal control.
The level of adiponectin is decreased but visfatin expression is increased in the MS patients when compared with the normal controls
To determine if the expression levels of adiponectin and visfatin were altered in the MS patients when compared with the normal control group, the total proteins were extracted from blood samples collected from the two groups. The protein levels were determined by immunoblotting. As indicated in Figure 1A, the levels of adiponectin were decreased in the MS groups when compared with the normal controls. However, the levels of visfatin were increased in the MS samples when compared with the normal controls. Representative western blots were given in Figure 1B. These results confirmed that levels of adiponectin are decreased but visfatin expression levels are increased in the MS patients when compared with the normal controls.
Figure 1.
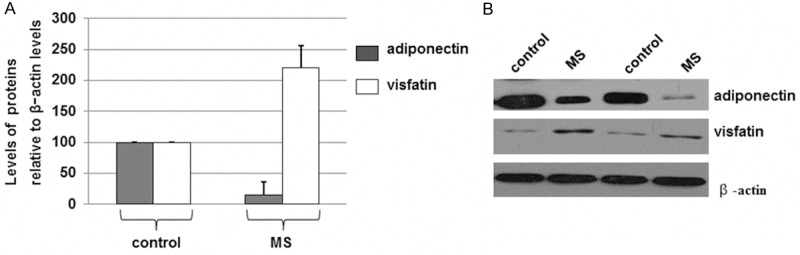
Western blot analyses of adiponectin and visfatin levels in MS patients and the control individuals with normal body weights. A. The total proteins were extracted from bloods, separated on SDS/PAGE gels, and determined by immunoblot analyses. The antibodies against adiponectin, visfatin, or β-actin were used. Histograms show the mean normalized optical density (OD) of the protein bands relative to the OD of β-actin band from the same individual. Error bars show SD (P < 0.05). B. Representative blots in the same experiments were shown.
The mRNA levels of adiponectin and visfatin expression are altered in the MS patients when compared with the normal controls
Many reasons may cause decreases in protein levels. Transcriptional regulation is an important reason. To determine if the altered protein levels of adiponectin and visfatin, the total RNAs were isolated and then determined by RT-PCR. The levels of the mRNA transcripts in controls were assigned a level of 10.
As indicated in Figure 2, the mean levels of adiponectin mRNA transcripts in MS patients were decreased when compared with the normal controls. However, the levels of visfatin were increased in the MS samples when compared with the normal controls. These results suggested that the levels of adiponectin are decreased but visfatin expression levels are increased in the MS patients when compared with the normal controls via a transcriptional mechanism.
Figure 2.
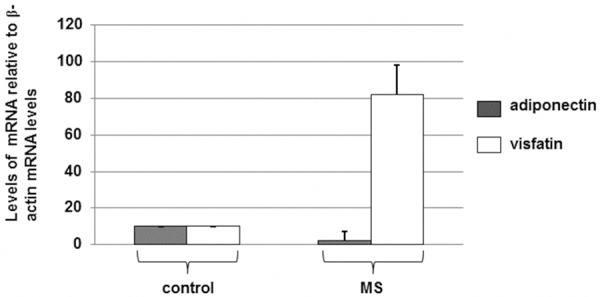
Quantitative RT-PCR analysis of the mRNA expression levels of adiponectin and visfatin levels in MS patients and the control individuals with normal body weights. RNA was extracted from bloods and reverse transcribed into cDNA using random primers. Expression levels of adiponectin, visfatin, and β-actin mRNAs were determined. The levels (mean value) of the transcripts in all individuals were calculated. Error bars show SD of the mean values (P < 0.05).
Discussion
MS has resulted in an epidemiological concern followed with the increasing prevalence of obesity in the world [14]. The MS patients have approximately 45% higher mortality and 78% higher coronary heart disease-related deaths in comparison to people without MS [15,16]. MS has also been found to be related with insulin resistance in livers [17].
In this study, we have compared the clinical characteristics of MS patients and the controls with normal body weights. We found that the expression levels of adiponectin and visfatin are significantly different between the MS group and the normal control (p < 0.05). Furthermore, the protein levels and mRNA levels of adiponectin and visfatin in blood collected from the two groups were compared. It has been found that levels of adiponectin are decreased but visfatin expression levels are increased in the MS patients when compared with the normal controls. Moreover, such alterations might be done via a transcriptional mechanism since the real-time PCR results suggested that the mRNA levels of adiponectin are decreased but visfatin mRNA levels are increased in the MS patients when compared with the normal controls.
Recently, several new factors related to MS are reported. For example, the visceral adiposity index, a recently discovered marker of cardiometabolic risk, was analyzed and found that the visceral adiposity index is a good marker of MS [18]. In addition, the increased FcgR expression level and the activity in monocytes of MS patients are correlated with the features of MS [19]. Our discovery that levels of adiponectin and visfatin may be used as diagnosis markers of metabolic syndrome in Uygur population will provide good basis for further study of MS.
Acknowledgements
This work was supported by the National Natural Science Foundation of China grant (No. 30960363).
Disclosure of conflict of interest
None.
References
- 1.Katsiki N, Athyros VG, Karagiannis A, Mikhailidis DP. Metabolic Syndrome and Non-Cardiac Vascular Diseases: an Update from Human Studies. Curr Pharm Des. 2014;20:4944–52. doi: 10.2174/1381612819666131206100750. [DOI] [PubMed] [Google Scholar]
- 2.Doyon A, Schaefer F. The prodromal phase of obesity-related chronic kidney disease: early alterations in cardiovascular and renal function in obese children and adolescents. Nephrol Dial Transplant. 2013;4:iv50–57. doi: 10.1093/ndt/gft263. [DOI] [PubMed] [Google Scholar]
- 3.Carbone F, Montecucco F, Mach F, Pontremoli R, Viazzi F. The liver and the kidney: two critical organs influencing the atherothrombotic risk in metabolic syndrome. Thromb Haemost. 2013;110:940–958. doi: 10.1160/TH13-06-0499. [DOI] [PubMed] [Google Scholar]
- 4.Baber U, Gutierrez OM, Levitan EB, Warnock DG, Farkouh ME, Tonelli M, Safford MM, Muntner P. Risk for recurrent coronary heart disease and all-cause mortality among individuals with chronic kidney disease compared with diabetes mellitus, metabolic syndrome, and cigarette smokers. Am Heart J. 2013;166:373–380. doi: 10.1016/j.ahj.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DT, Watts GF, Irish AB, Ooi EM, Dogra GK. Insulin resistance and the metabolic syndrome are associated with arterial stiffness in patients with chronic kidney disease. Am J Hypertens. 2013;26:1155–1161. doi: 10.1093/ajh/hpt077. [DOI] [PubMed] [Google Scholar]
- 6.Sisson SB, Shay CM, Camhi SM, Short KR, Whited T. Sitting and cardiometabolic risk factors in U. S. Adolescents. J Allied Health. 2013;2:236–242. [PubMed] [Google Scholar]
- 7.Creydt VP, Sacca PA, Tesone AJ, Vidal L, Calvo JC. Adipocyte differentiation influences the proliferation and migration of normal and tumoral breast epithelial cells. Mol Med Rep. 2010;3:433–439. doi: 10.3892/mmr_00000276. [DOI] [PubMed] [Google Scholar]
- 8.Xiao H, Shan L, Zhu H, Xue F. Detection of significant pathways in osteoporosis based on graph clustering. Mol Med Rep. 2012;6:1325–1332. doi: 10.3892/mmr.2012.1082. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, Hulver M, McMillan RP, Cai L, Kershaw EE, Yu L, Xue B, Shi H. Regulation of insulin and leptin signaling by muscle suppressor of cytokine signaling 3 (SOCS3) PLoS One. 2012;7:e47493. doi: 10.1371/journal.pone.0047493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mena A, Pedreira JD, Castro A, López S, Vázquez P, Poveda E. Metabolic Syndrome Is Associated With Fibrosis Development In Chronic Hepatitis B Virus Inactive Carriers. J Gastroenterol Hepatol. 2014;29:173–178. doi: 10.1111/jgh.12432. [DOI] [PubMed] [Google Scholar]
- 11.Negro F. HCV infection and metabolic syndrome: which is the chicken and which is the egg? Gastroenterology. 2012;142:1288–1292. doi: 10.1053/j.gastro.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 12.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2:133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu YC, Hsu CC, Yu TH, Wang CP, Lu LF, Hung WC, Chiu CA, Chung FM, Lee YJ, Tsai IT. Association between visfatin levels and coronary artery disease in patients with chronic kidney disease. Iran J Kidney Dis. 2013;7:446–452. [PubMed] [Google Scholar]
- 14.James PT. Obesity: the worldwide epidemic. Clin Dermatol. 2004;22:276–280. doi: 10.1016/j.clindermatol.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Gami AS, Witt BJ, Howard DE, Erwin PJ, Gami LA, Somers VK, Montori VM. Metabolic syndrome and risk of incident cardiovascular events and deatha systematic review and meta-analysis of longitudinal studies. J Am Coll Cardiol. 2007;49:403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 16.Hui WS, Liu Z, Ho SC. Metabolic syndrome and all-cause mortality: a meta-analysis of prospective cohort studies. Eur J Epidemiol. 2010;25:375–384. doi: 10.1007/s10654-010-9459-z. [DOI] [PubMed] [Google Scholar]
- 17.Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 18.Mazzuca E, Battaglia S, Marrone O, Marotta AM, Castrogiovanni A, Esquinas C, Barcelò A, Barbé F, Bonsignore MR. Gender-specific anthropometric markers of adiposity, metabolic syndrome and visceral adiposity index (VAI) in patients with obstructive sleep apnea. J Sleep Res. 2014;23:13–21. doi: 10.1111/jsr.12088. [DOI] [PubMed] [Google Scholar]
- 19.Devaraj S, Chen X, Adams-Huet B, Jialal I. Increased expression of Fc-γ receptors on monocytes in patients with nascent metabolic syndrome. J Clin Endocrinol Metab. 2013;98:E1510–1515. doi: 10.1210/jc.2013-2112. [DOI] [PubMed] [Google Scholar]


