Figure 1.
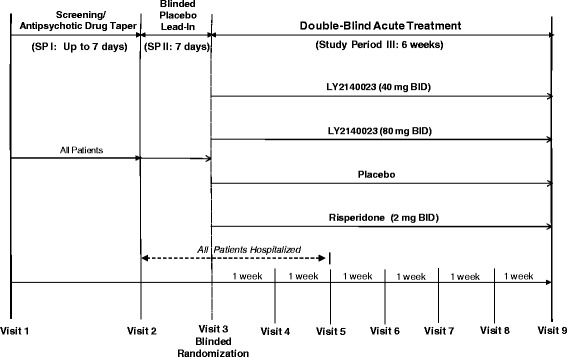
The unblinded study design. Study HBBM consisted of 3 periods: a screening phase, a 7-day placebo lead-in phase that was blinded to investigators and patients, and a 6-week randomized treatment phase. All patients were hospitalized from Visits 2–5. Patients could be discharged after that time based upon clinical presentation.
