Supplemental Digital Content is available in the text.
Keywords: pneumonia, HIV, AIDS, children, lower respiratory tract infection, South Africa
Abstract
Background:
Data on the epidemiology of viral-associated acute lower respiratory tract infection (LRTI) from high HIV prevalence settings are limited. We aimed to describe LRTI hospitalizations among South African children aged <5 years.
Methods:
We prospectively enrolled hospitalized children with physician-diagnosed LRTI from 5 sites in 4 provinces from 2009 to 2012. Using polymerase chain reaction (PCR), nasopharyngeal aspirates were tested for 10 viruses and blood for pneumococcal DNA. Incidence was estimated at 1 site with available population denominators.
Results:
We enrolled 8723 children aged <5 years with LRTI, including 64% <12 months. The case-fatality ratio was 2% (150/8512). HIV prevalence among tested children was 12% (705/5964). The overall prevalence of respiratory viruses identified was 78% (6517/8393), including 37% rhinovirus, 26% respiratory syncytial virus (RSV), 7% influenza and 5% human metapneumovirus. Four percent (253/6612) tested positive for pneumococcus. The annual incidence of LRTI hospitalization ranged from 2530 to 3173/100,000 population and was highest in infants (8446–10532/100,000). LRTI incidence was 1.1 to 3.0-fold greater in HIV-infected than HIV-uninfected children. In multivariable analysis, compared to HIV-uninfected children, HIV-infected children were more likely to require supplemental-oxygen [odds ratio (OR): 1.3, 95% confidence interval (CI): 1.1–1.7)], be hospitalized >7 days (OR: 3.8, 95% CI: 2.8–5.0) and had a higher case-fatality ratio (OR: 4.2, 95% CI: 2.6–6.8). In multivariable analysis, HIV-infection (OR: 3.7, 95% CI: 2.2–6.1), pneumococcal coinfection (OR: 2.4, 95% CI: 1.1–5.6), mechanical ventilation (OR: 6.9, 95% CI: 2.7–17.6) and receipt of supplemental-oxygen (OR: 27.3, 95% CI: 13.2–55.9) were associated with death.
Conclusions:
HIV-infection was associated with an increased risk of LRTI hospitalization and death. A viral pathogen, commonly RSV, was identified in a high proportion of LRTI cases.
In 2010, an estimated 1.4 million children died due to acute lower respiratory tract infections (LRTI) and an estimated 11.9 million were hospitalized1 H,2IV infection is associated with increased severity of LRTI and higher case-fatality ratiosT3–5he relative contribution of viral and bacterial etiologies to the syndrome of LRTI also varies by HIV status6 D,7ata from Europe and North America suggest that even in the presence of widespread availability of highly active antiretroviral therapy (HAART), the incidence of LRTI remains elevated in HIV-infected children8,9
There are limited data on the burden, etiology and epidemiology of LRTI in HIV-infected and HIV-uninfected children in sub-Saharan Africa in the era of HAART availability. In South Africa among children aged <5 years in 2011, the estimated HIV prevalence was 4% and coverage with HAART was approximately 26%10Haemophilus influenzae type b conjugate vaccine was introduced into the South African immunization program in 1999 and the pneumococcal conjugate vaccine (PCV) in 2009A11s bacterial etiologies decline due to vaccination, respiratory viral causes of LRTI may gain greater prominence. We aimed to describe the incidence, etiology and factors associated with death among HIV-infected and HIV-uninfected children aged <5 years hospitalized with LRTI in South Africa from 2009 through 2012.
MATERIALS AND METHODS
Description of the Surveillance Program
From February 2009, active, prospective, hospital-based surveillance [the Severe Acute Respiratory Illness (SARI) program] was implemented in 3 of the 9 provinces of South Africa [Chris Hani-Baragwanath Academic Hospital (CHBAH) in an urban area of Gauteng Province, Edendale Hospital in a periurban area of KwaZulu-Natal Province and Matikwana and Mapulaneng Hospitals in a rural area of Mpumalanga Province]. In June 2010, an additional surveillance site was introduced at Klerksdorp Hospital in a periurban area of the Northwest Province12
Case Definition
A case of hospitalized-LRTI was defined as a hospitalized child with <7 days symptom duration meeting age-appropriate clinical case definitions (aged 2 days through <3 months of age with physician diagnosis of LRTI, including bronchitis, bronchiolitis, pneumonia and/or pleural effusion).
Study Procedures
All patients admitted from Monday through Friday were eligible. The total number of children admitted meeting study case definitions and numbers enrolled were documented for the entire study-period. Study staff completed case report forms until discharge and collected nasopharyngeal aspirates and blood specimens. All decisions on medical-care, including mechanical ventilation and other investigations such as blood bacteria culture, testing for Mycobacterium tuberculosis and CD4+ T-lymphocyte counts was undertaken at the discretion of the attending-physician. At CHBAH, children with LRTI but without World Health Organization signs of severe LRTI and who do not require supplemental-oxygen treatment may be admitted to a short stay ward for 1–2 days, following which they are either discharged or admitted to the general pediatric wards. Children in the short stay wards were included. Data in case report forms were reviewed regularly to identify inconsistencies and regular site visits conducted to ensure adherence to study procedures.
Evaluation of HIV Sero-status
HIV-infection status was determined based on testing undertaken as part of standard-of-careo13r through anonymized linked dried blood spot specimen testing by HIV PCR for children aged <18 months and by enzyme-linked immunosorbent assay for individuals aged ≥18 months. CD4+ T-cell counts were determined by flow cytometryP14atients were categorized into 2 immunosuppression categories such as (1) severe immunosuppression (CD4+ T-lymphocytes <25% among infants, CD4+ <20% among 12–35 months children or CD4+ <15% among those 36–59 months age) or (2) mild or no immunosuppression15
Laboratory Methods
Nasopharyngeal aspirates were immersed in viral transport medium at 4–8°C and transported to the National Institute for Communicable Diseases (NICD) within 72 hours of collection. Specimens were tested by multiplex real-time reverse-transcription PCR assay for 10 respiratory viruses including influenza A and B viruses, parainfluenza virus I-III (PIV), respiratory syncytial virus (RSV), enterovirus, human metapneumovirus (hMPV), adenovirus and human rhinovirus (hRV)O16wing to challenges with availability of reagents, we did not test for adenovirus from August to October 2009. Streptococcus pneumoniae was identified by quantitative real-time PCR detecting the lytA gene from whole blood specimens17
Calculation of Incidence
Incidence estimates were calculated for Soweto (CHBAH) for which population denominator data were available. This hospital is the only public hospital serving a community of about 120,000 children aged <5 years in 2012 among whom <10% have private medical insurance. Consequently, many of individuals requiring hospitalization from this community are admitted to CHBAH. We estimated the incidence of hospitalizations per 100,000 individuals using the number of LRTI hospitalizations, adjusting for nonenrollment (refusal to participate and nonenrollment during weekends) by age groups and HIV status divided by the mid-year total population estimatef18or each year, multiplied by 100,000. HIV prevalence in the study population was estimated from the projections of the Actuarial Society of South Africa AIDS and Demographic modelT10hese estimates have been validated by comparison with other estimates and compare favorably with estimates from population-based HIV prevalence studiesW19e assumed that the HIV prevalence by age group among children not tested for HIV was the same as those tested. Confidence intervals for incidence estimates were calculated using the Poisson distribution. Age-specific and overall age-adjusted risk of hospitalization in HIV-infected and HIV-uninfected persons was determined using log-binomial regression. To explore the possible effect of missing data on estimates of HIV-specific incidence, a sensitivity analysis was conducted in which all cases not tested for HIV were assumed to be HIV-uninfected.
Analysis of Factors Associated With HIV Infection and Death
Univariate and multivariable analyses were performed with Stata version 12 (StataCorp Limited, College Station, TX). To identify factors associated with HIV status and death, we implemented multivariable logistic regression models, starting with all variables that were significant at P < 0.1 on univariate analysis, and dropping nonsignificant factors with stepwise backward selection. All 2-way interactions were evaluated. Two-sided P-values <0.05 were considered significant throughout. For each univariate analysis, we used all available case information. In the multivariable model, patients with missing data for included variables were dropped from the model. The Hosmer–Lemeshow test was used to evaluate the fit of the model to the data; a P > 0.40 is considered a good fit of the model to the data. Age group, duration of hospitalization and year were defined as categorical variables with multiple levels. All other variables were defined as the presence or absence of the attribute excluding missing data. To explore possible bias, patients tested for HIV were compared with those not tested.
Ethical Considerations
The protocol was approved by the Research Ethics Committees of the Universities of the Witwatersrand and KwaZulu-Natal. This surveillance was deemed nonresearch by the US CDC.
RESULTS
Demographic and Clinical Characteristics
From February 2009 through December 2012, 13,815 children <5 years of age fulfilling the LRTI case definition were screened for study enrollment, of whom 8723 (63%) were enrolled. The most common reasons for nonenrollment were study refusal (n = 1209, 24%) or unavailability of a legal guardian (n = 1452, 29%). Of the children enrolled, 64% (5603) were <12 months age, and 69% (6024) were enrolled at CHBAH (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B963).
HIV infection status was determined in 5964 (68%) enrolled children. The proportion of children with available HIV results increased from 48% in 2009 to 77% in 2012 (P < 0.001). The overall HIV prevalence was 12% (705/5964) and varied by age group: 10% (249/2565) in those <6 months, 13% (163/1290) in 6–11 months, 10% (125/1201) in 12–23 months and 19% (168/908) in 24–59 months (P < 0.001). HIV prevalence varied by hospital: 8% (322/3893) at CHBAH, 23% (206/906) at Matikwana and Mapulaneng, 14% (118/850) at Edendale and 19% (59/315) at Klerksdorp (P < 0.001). At CHBAH, the HIV prevalence among children admitted to the short stay ward was 5% (97/1965) compared with 12% (225/1928) among those admitted to the general pediatric wards (P < 0.001).
Only 2% of children reported an underlying medical condition other than HIV-infection (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B963). The case-fatality ratio was 2% (150/8512) and 1% (109/8634) were admitted to the intensive care unit. Supplemental-oxygen was administered to 35% (3042/8637) of children (63%, 277/437 at Klerksdorp; 62%, 661/1062 at Edendale; 21%, 246/1181 at Matikwana and Mapulaneng; 59%, 1746/2932 in the CHBAH general pediatric wards and 4%, 115/3025 at the CHBAH short stay ward). Data on presence or absence of World Health Organisation criteria for clinical severity of pneumoniw20ere only available for 44% (3841/8723) of children, of whom 23% (900/3841) would be classified as having pneumonia, 23% (894/3841) severe pneumonia and 53% (2047/3841) very severe pneumonia. All children were eligible to receive H. influenzae type b conjugate vaccine and 5292 to receive PCV. Coverage of the primary schedule of H. influenzae type b conjugate vaccine was 78% (4477/5719) and PCV was 46% (1745/3765) among eligible children with available data. Only 6 patients reported having received the influenza vaccine.
Incidence of Hospitalization in HIV-infected and HIV-uninfected Patients
The annual incidence per 100,000 population of LRTI hospitalization among children <5 years at CHBAH ranged from 2530 to 3173, being highest in infants (annual range: 8446–10,532; Table 1). In addition, the incidence per 100,000 population was higher in children <4 months age (15,573–21,752) than those aged 4–11 months (5424–6792). Among children <4 months age, the incidence per 100,000 population was higher in HIV-infected (27,336–49,966) than HIV-uninfected children (15,044–21,565). Similarly in children aged 4–11 months, the incidence per 100,000 population was higher in HIV-infected (8628–34,152) than HIV-uninfected children (5105–6730).
TABLE 1.
Incidence of Acute LRTI Hospitalizations per 100,000 Population by Year and HIV Status Among Children <5 Years in Soweto, as Measured at Chris Hani-Baragwanath Hospital, South Africa
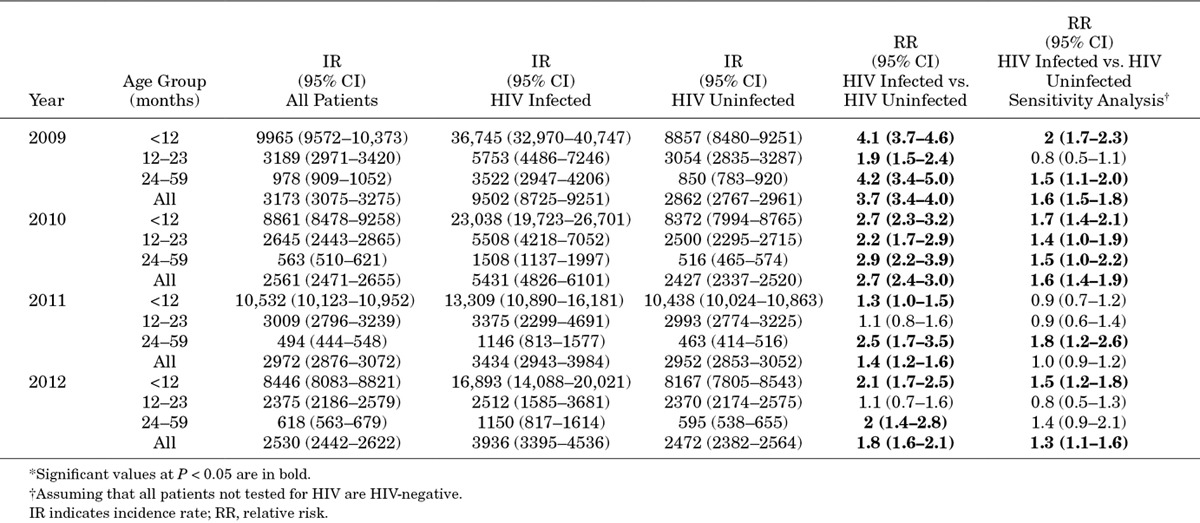
HIV-infected children had up to 3-fold greater incidence of LRTI compared with uninfected children, although the relative risk of hospitalization among HIV-infected individuals decreased in later years (Table 1). On sensitivity analysis, assuming that all patients not tested for HIV were HIV-uninfected, the trend toward a higher incidence of LRTI hospitalizations in HIV-infected compared with uninfected children remained in most age groups and most years, but relative risks were lower in more recent years.
Etiologic Agents Identified
Overall, 78% of LRTI episodes was associated with a viral infection, including 37% for hRV, 26% for RSV, 26% for adenovirus, 10% for enterovirus and other individual viruses in <10% of children (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B963). Thirty-three percent (2893/8723) of patients had sterile site specimens (2890 blood culture, 2 pleural fluid culture and 1 blood and pleural fluid culture) submitted for bacterial culture. Two percent (75/3196) of patients had a positive bacterial culture (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B963). Only 37 patients had laboratory-confirmed tuberculosis. Four percent (253/6612) of children had pneumococcal infection. Among patients testing positive for pneumococcus, 76% (187/245) also tested positive for at least 1 respiratory virus, similar to the percent testing positive for respiratory viruses among pneumococcus-negative patients (76%, 4703/6156). The only virus with a significantly different prevalence among pneumococcal-positive compared with pneumococcal-negative children was influenza (13%, 31/245 detection among pneumococcus-positive vs. 7%, 432/6157 among pneumococcus-negative children, P = 0.001). Patients with very severe pneumonia were more likely to test positive for influenza [6% (48/859) vs. 6% (51/862) vs. 8% (156/1969) in pneumonia, severe and very severe pneumonia respectively, P = 0.03]. There was no association between pneumonia severity and any other pathogen tested.
When the proportion of patients testing positive for different pathogens was analyzed by age group, the prevalence of infection increased with increasing age for influenza, adenovirus, enterovirus, rhinovirus and pneumococcus, while RSV was most common in the 0–3 month age group (Table 2). The proportion of patients testing positive for individual or any respiratory viruses did not differ by pneumococcal vaccination status (results not shown). LRTI cases were enrolled throughout the year with peaks in late summer and autumn concomitant with RSV circulation and winter and spring associated with influenza circulation (Fig. 1).
TABLE 2.
Percentage of Patients Testing Positive for Viral and Bacterial Pathogens by Age Group Among Children <5 Years Hospitalized With Acute LRTI at 4 Sentinel Surveillance Sites, South Africa, 2009–2012
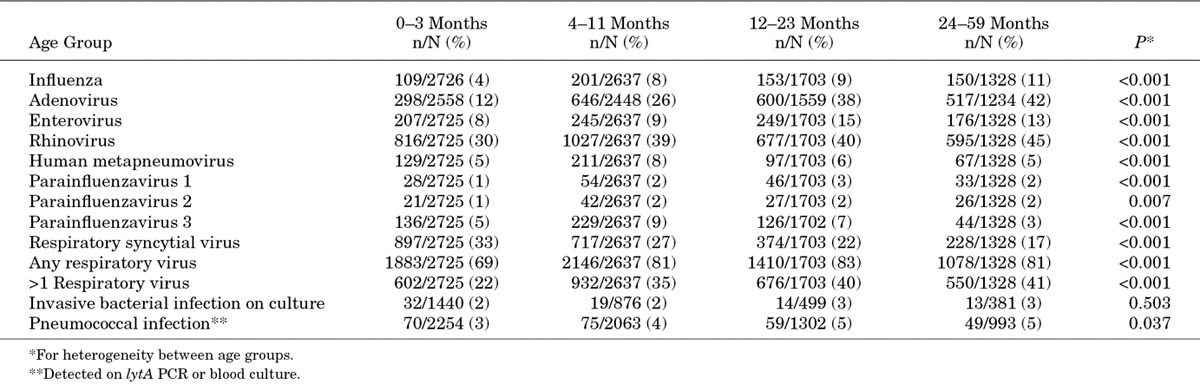
FIGURE 1.
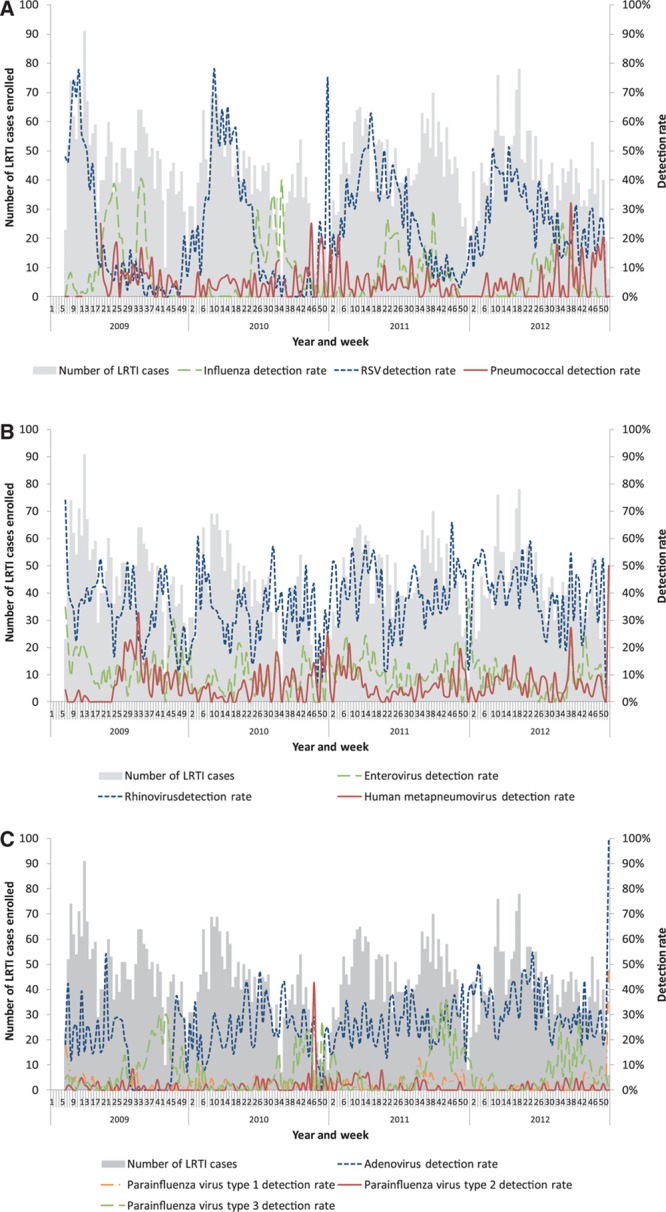
Number of patients enrolled with LRTI and detection rates by epidemiologic week and year at 4 sentinel surveillance sites, South Africa, 2009–2011. (A) Influenza, RSV and pneumococcal detection rates. (B) Enterovirus, rhinovirus and human metapneumovirus detection rates. (C) Adenovirus and parainfluenza virus 1, 2 and 3 detection rates.
Characteristics of HIV-infected Children and Factors Associated With HIV Infection
Compared to children without HIV infection, using multivariable analysis controlling for year and hospital, HIV-infected children were more likely to be in the older 24–59 month age group, have symptoms ≥2 days before admission, require supplementary-oxygen, be hospitalized for longer duration and to die (see Table, Supplemental Digital Content 1, http://links.lww.com/INF/B963). In contrast, they were less likely to be infected with RSV or hMPV.
Only 25% (174/705) of HIV-infected patients had available CD4+ T-lymphocyte data, of whom 64% had severe immunosuppressionF15orty-four percent (96/217) of those with available data reported currently receiving HAART and 30% (207/690) reported receiving prophylaxis with trimethoprim–sulfamethoxazole.
Factors Associated With In-hospital Mortality Among Children Hospitalized With LRTI
The unadjusted case-fatality ratio was greater among HIV-infected (47/695, 7%) compared with HIV-uninfected (46/5240, 1%) children (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B964). On multivariable analysis, controlling for year and site, HIV-positivity, pneumococcal infection, receipt of mechanical ventilation and receipt of supplementary-oxygen were independent risk indicators associated with death, while case-fatality ratio was lower in children who tested RSV-positive (see Table, Supplemental Digital Content 2, http://links.lww.com/INF/B964). The case-fatality ratio was not significantly different between patients with (8%, 9/112) and without (3%, 2/61) severe immunosuppression, (P = 0.221) or patients receiving (3%, 3/95) and not receiving (8%, 9/118) HAART (P = 0.160) although numbers were small.
DISCUSSION
HIV-infected children have an elevated incidence of LRTI hospitalization, duration of hospitalization and in-hospital case-fatality ratio compared with HIV-uninfected children in South Africa. This indicates that underlying HIV-infection remains a major risk factor for pneumonia morbidity and mortality in settings such as ours, even following HAART availability. Of the respiratory viruses with a likely pathogenic role, RSV was identified in more than 25% of children, particularly in infants, and influenza was identified in 7% of children. While pneumococcus was not as commonly identified (4% of cases), likely related to lower sensitivity of available diagnostic assays for pneumococcal pneumonia, infection with pneumococcus was an independent risk factor for death.
The overall incidence of hospitalization per 100,000 population for LRTI in Soweto among children <5 years of age (~2500–3000) was similar to that described from Africa in a recent systematic review of global pneumonia burden (~2260, 95% CI: 1530–3340)W2e observed the highest incidence in those aged <12 months (~8000–11,000/100,000 population), where incidences were slightly higher than other estimates for Africa (~5080/100,000 population, 95% CI: 3360–7690/100,000) but similar to those from some studies from middle-income countries2,21,22
Pneumonia is the commonest reason for hospitalization among African HIV-infected childrenI6n our study, HIV-infected children experienced an elevated incidence of hospitalization compared with HIV-uninfected children but this excess risk decreased over the study period from 3.7 (95% CI: 3.4–4.0) in 2009 to 1.8 (95% CI: 1.6–2.1) in 2012, possibly related to more widespread availability of early HAART over this period23 S,24tudies have shown that HAART availability is associated with a reduction in pneumonia incidence in HIV-infected children, albeit HIV-infected children on HAART still have a higher incidence of pneumonia3,8 W,9e found that HIV-infected children experienced a 4 times increased risk of death compared with HIV-uninfected children once hospitalized. This is similar to several other studies which reported case-fatality ratios 3–6 times greater in HIV-infected children6,7,25 T,26he overall case-fatality ratios among HIV-infected children in our study are, however, lower than has previously been described in HIV-infected children from South Africa, possibly related to more widespread HAART availability.
HRV (37%), RSV (26%) and adenovirus (26%) were the most commonly identified respiratory viruses, while influenza was identified in 7% of patients, similar to what has been found in other studies from Africa using PCR for viral diagnosis22 C,27–29hallenges remain for the attribution of a causal role for some viruses (including hRV, adenovirus and enterovirus), which are also identified at high frequency from healthy individuals22,27 R,29SV and hMPV (both thought to have a likely pathogenic role) were identified less frequently in HIV-infected than HIV-uninfected children suggesting that the increased risk of hospitalization due to HIV-associated immunosuppression is relatively lower for these pathogens than for other etiologies. Previous studies from South Africa have shown that while the detection rate for viruses may be lower in HIV-infected children, the incidence of hospitalization is substantially higher in HIV-infected compared with HIV-uninfected children7 C,30hildren with RSV were less likely to die, as has been previously describedT22his is likely because the relative contribution of viral etiologies compared with bacterial etiologies is greater for hospitalized pneumonia than among deaths 31high proportion of children were coinfected with multiple respiratory viruses (33%), as has been described previously, although coinfection was not associated with adverse outcome16,29 H,32IV-infected children have been described to have an increased risk of polymicrobial infections due to multiple bacterial, viral and fungal pathogensW3e were not able to assess this as we did not systematically test for important pathogens in HIV-infected children, particularly tuberculosis, Pneumocystis jiroveci and cytomegalovirus.
Blood cultures were performed in less than half of children, limiting our ability to comment on the proportion with bacteremic pneumonia, but similar to other studies from South Africa, the pneumococcus and Staphylococcus aureus were the most common bacteria identified. S. pneumoniae is an important cause of pneumonia in HIV-infected and HIV-uninfected children25 I,26n our study, we identified pneumococcus from 4% of children overall using a combination of PCR and blood cultures (performed in a minority of patients). These rates are similar to other studies from South Africa before PCV introduction which used systematic blood culture collection only25 F,26ailure to observe lower detection rates after PCV introduction may be attributed to our diagnostic technique, real-time PCR, identifying more pneumococcal cases than blood culture in our settingN33otwithstanding this, additional cases of pneumococcal coinfection may still have been missed34 P,35neumococcal DNA in the blood may reflect occult bacteremia in some individualsP36–38atients testing positive for pneumococcus had a high prevalence of detection of respiratory viruses although many of these may have been bystander virusesI32nfluenza was the only virus to be more commonly detected in patients testing positive for pneumococcus. The association between influenza and pneumococcal infection has been described previously12,39
Our study had potential limitations. HIV status data was only available for approximately 60% of children. If the characteristics of untested children differed from those of tested children this may have biased findings; however, we did not find any significant differences in epidemiologic characteristics of tested and untested patients (results not shown). In addition, for the estimation of incidence stratified by HIV status, we conducted a sensitivity analysis to explore the scenario if all untested children were HIV-uninfected. Data on access to HAART and CD4+ T-cell count were only available for a limited number of HIV-infected children limiting our ability to comment on the effect of HAART and degree of immunocompromise on disease incidence and severity. Incidence data were only available for 1 urban site with available denominator data and thus may not be generalizable to other settings within South Africa. We may have underestimated incidence as not all cases may have sought care in hospital. Similarly, patients who die may be less likely to be enrolled into studies such as ours and this may lead to underestimation of case-fatality ratios and potential exclusion of most severely ill children 27The denominators for the numbers of HIV-infected and HIV-uninfected children each year were obtained from the 2008 version of the Actuarial Society of South Africa model. Subsequent to the development of this model, rates of access to prevention of mother-to-child transmission and early HAART among children have been more rapid than expected leading to possible overestimation of the numbers of children with AIDS in South Africa, which could have led to an underestimation of the relative risk of hospitalization in HIV-infected children10,24There were a large number of statistical analyses conducted on these data and corrections were not made for multiple testing.
In conclusion, we have demonstrated a high incidence of hospitalization 1 for LRTI amongst children aged <5 years in South Africa, particularly in children aged <1 year and HIV-infected children. HIV-infected children also experience increased hospitalization duration and mortality. RSV was an important cause of pneumonia. Vaccines for RSV are currently under development and could be expected to have a substantial impact on pneumonia burden if they become available.40 Ongoing reductions in burden of pneumococcal pneumonia may be expected following PCV introduction. Influenza was identified in 7% of children and influenza vaccination is recommended for children <5 years in South Africa but very few children in our study reported being vaccinated.41 Influenza vaccines are less effective in children <5 years than healthy adults and efficacy has not been demonstrated in HIV-infected children.42 More widespread access to influenza vaccines could, however, potentially impact disease burden, particularly if newer formulations such as adjuvanted vaccines are shown to be safe and effective in our setting. HIV remains a major risk factor for LRTI and adverse outcome, and more widespread adherence to PMTCT protocols and availability of HAART can be expected to have ongoing impact in reducing disease burden.3,6
Footnotes
The authors have no conflicts of interest to disclose.
This study received funding from the National Institute for Communicable Diseases of the National Health Laboratory Service and was supported in part by funds from the United States Centers for Disease Control and Prevention (CDC), Atlanta, Georgia Preparedness and Response to Avian and Pandemic Influenza in South Africa (Cooperative Agreement Number: U51/IP000155-04). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. The funders had no role in study design, implementation, manuscript writing or the decision to submit for publication. The corresponding author had full access to all the data in the study and takes final responsibility for the decision to submit for publication.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.Nair H, Simooes EA, Rudan I, et al. Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet. 2013:10–6736. doi: 10.1016/S0140-6736(12)61901-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray DM, Zar HJ. Community-acquired pneumonia in HIV-infected children: a global perspective. Curr Opin Pulm Med. 2010;16:208–216. doi: 10.1097/MCP.0b013e3283387984. [DOI] [PubMed] [Google Scholar]
- 4.Jaspan HB, Huang LC, Cotton MF, et al. Bacterial disease and antimicrobial susceptibility patterns in HIV-infected, hospitalized children: a retrospective cohort study. PLoS One. 2008;3:e3260. doi: 10.1371/journal.pone.0003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNally LM, Jeena PM, Gajee K, et al. Effect of age, polymicrobial disease, and maternal HIV status on treatment response and cause of severe pneumonia in South African children: a prospective descriptive study. Lancet. 2007;369:1440–1451. doi: 10.1016/S0140-6736(07)60670-9. [DOI] [PubMed] [Google Scholar]
- 6.Zar HJ, Madhi SA. Childhood pneumonia–progress and challenges. S Afr Med J. 2006;96(9, pt 2):890–900. [PubMed] [Google Scholar]
- 7.Madhi SA, Schoub B, Simmank K, et al. Increased burden of respiratory viral associated severe lower respiratory tract infections in children infected with human immunodeficiency virus type-1. J Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 8.Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 9.Chiappini E, Galli L, Tovo PA, et al. Changing patterns of clinical events in perinatally HIV-1-infected children during the era of HAART. AIDS. 2007;21:1607–1615. doi: 10.1097/QAD.0b013e32823ecf5b. [DOI] [PubMed] [Google Scholar]
- 10.Actuarial Society of South Africa (ASSA) AIDS and Demographic model. 2014.;27 http://aids.actuarialsociety.org.za/ASSA2008-Model-3480.htm. [Google Scholar]
- 11.Madhi SA, Cohen C, von Gottberg A. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: translating research into policy. Vaccine. 2012;30(suppl 3):C21–C27. doi: 10.1016/j.vaccine.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 12.Cohen C, Moyes J, Tempia S, et al. Severe influenza-associated respiratory infection in high HIV prevalence setting, South Africa, 2009-2011. Emerg Infect Dis. 2013;19:1766–1774. doi: 10.3201/eid1911.130546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh E, Cohen C, Govender N, Meiring S. A description of HIV testing strategies at 21 laboratories in South Africa. Commun Dis Surveill Bull. 2008;6:16–17. [Google Scholar]
- 14.Glencross D, Scott LE, Jani IV, et al. CD45-assisted PanLeucogating for accurate, cost-effective dual-platform CD4+ T-cell enumeration. Cytometry. 2002;50:69–77. doi: 10.1002/cyto.10068. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organisation HIV/AIDS Programme. WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunologic Classification of HIV-related Disease in Adults and Children. Geneva, Switzerland: World Health Organisation; 2007. [Google Scholar]
- 16.Pretorius MA, Madhi SA, Cohen C, et al. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness–South Africa, 2009-2010. J Infect Dis. 2012;206(suppl 1):S159–S165. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- 17.Carvalho MG, Tondella ML, McCaustland K, et al. Evaluation and improvement of real-time PCR assays targeting lytA, ply, and psaA genes for detection of pneumococcal DNA. J Clin Microbiol. 2007;45:2460–2466. doi: 10.1128/JCM.02498-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Statistics South Africa. STATSSA, Mid-year Population Estimates 2003–2008. Pretoria, South Africa: Statistics South Africa; 2008. [Google Scholar]
- 19.Shisana O, Rehle T, Simbayi LC, et al. the SABSSM III Implementation Team. South African National HIV Prevalence, Incidence, Behaviour and Communication Survey, 2008: A Turning Tide Among Teenagers? Cape Town, South Africa:: HSRC Press; 2009. [Google Scholar]
- 20.World Health Organisation. Handbook IMCI. Geneva, Switzerland: World Health Organisation; 2000. Integrated management of childhood illness. [Google Scholar]
- 21.Olsen SJ, Laosiritaworn Y, Siasiriwattana S, et al. The incidence of pneumonia in rural Thailand. Int J Infect Dis. 2006;10:439–445. doi: 10.1016/j.ijid.2006.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berkley JA, Munywoki P, Ngama M, et al. Viral etiology of severe pneumonia among Kenyan infants and children. JAMA. 2010;303:2051–2057. doi: 10.1001/jama.2010.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson LF, Davies MA, Moultrie H, et al. The effect of early initiation of antiretroviral treatment in infants on pediatric AIDS mortality in South Africa: a model-based analysis. Pediatr Infect Dis J. 2012;31:474–480. doi: 10.1097/INF.0b013e3182456ba2. [DOI] [PubMed] [Google Scholar]
- 24.Johnson LF. Access to antiretroviral treatment in South Africa, 2004–2011. South Afr J HIV Med. 2012;13:22–27. [Google Scholar]
- 25.Madhi SA, Petersen K, Madhi A, et al. Increased disease burden and antibiotic resistance of bacteria causing severe community-acquired lower respiratory tract infections in human immunodeficiency virus type 1-infected children. Clin Infect Dis. 2000;31:170–176. doi: 10.1086/313925. [DOI] [PubMed] [Google Scholar]
- 26.Zar HJ, Hanslo D, Tannenbaum E, et al. Aetiology and outcome of pneumonia in human immunodeficiency virus-infected children hospitalized in South Africa. Acta Paediatr. 2001;90:119–125. [PubMed] [Google Scholar]
- 27.Hammitt LL, Kazungu S, Morpeth SC, et al. A preliminary study of pneumonia etiology among hospitalized children in Kenya. Clin Infect Dis. 2012;54(suppl 2):S190–S199. doi: 10.1093/cid/cir1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Callaghan-Gordo C, Díez-Padrisa N, Abacassamo F, et al. Viral acute respiratory infections among infants visited in a rural hospital of southern Mozambique. Trop Med Int Health. 2011;16:1054–1060. doi: 10.1111/j.1365-3156.2011.02811.x. [DOI] [PubMed] [Google Scholar]
- 29.Feikin DR, Njenga MK, Bigogo G, et al. Viral and bacterial causes of severe acute respiratory illness among children aged less than 5 years in a high malaria prevalence area of western Kenya, 2007-2010. Pediatr Infect Dis J. 2013;32:e14–e19. doi: 10.1097/INF.0b013e31826fd39b. [DOI] [PubMed] [Google Scholar]
- 30.Moyes J, Cohen C, Pretorius M, et al. Epidemiology of respiratory syncytial virus-associated acute lower respiratory tract infection hospitalizations among HIV-infected and HIV-uninfected South African children, 2010–2011. J Infect Dis. 2013;208(suppl 3):S217–S226. doi: 10.1093/infdis/jit479. [DOI] [PubMed] [Google Scholar]
- 31.Rudan I, O’Brien KL, Nair H, et al. Epidemiology and etiology of childhood pneumonia in 2010: estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. J Glob Health. 2013;3:10401. doi: 10.7189/jogh.03.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruuskanen O, Lahti E, Jennings LC, et al. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolter N, Cohen C, Tempia S, et al. HIV and influenza are associated with increased blood pneumococcal load, South Africa, 2009–2011. J Infect Dis. 2013;209(1):56–65. doi: 10.1093/infdis/jit427. [DOI] [PubMed] [Google Scholar]
- 34.Madhi SA, Kuwanda L, Cutland C, et al. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin Infect Dis. 2005;40:1511–1518. doi: 10.1086/429828. [DOI] [PubMed] [Google Scholar]
- 35.Resti M, Moriondo M, Cortimiglia M, et al. Italian Group for the Study of Invasive Pneumococcal Disease. Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real-time polymerase chain reaction using blood samples. Clin Infect Dis. 2010;51:1042–1049. doi: 10.1086/656579. [DOI] [PubMed] [Google Scholar]
- 36.Azzari C, Moriondo M, Indolfi G, et al. Molecular detection methods and serotyping performed directly on clinical samples improve diagnostic sensitivity and reveal increased incidence of invasive disease by Streptococcus pneumoniae in Italian children. J Med Microbiol. 2008;57(pt 10):1205–1212. doi: 10.1099/jmm.0.2008/000935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rouphael N, Steyn S, Bangert M, et al. Use of 2 pneumococcal common protein real-time polymerase chain reaction assays in healthy children colonized with Streptococcus pneumoniae. Diagn Microbiol Infect Dis. 2011;70:452–454. doi: 10.1016/j.diagmicrobio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 38.Dagan R, Shriker O, Hazan I, et al. Prospective study to determine clinical relevance of detection of pneumococcal DNA in sera of children by PCR. J Clin Microbiol. 1998;36:669–673. doi: 10.1128/jcm.36.3.669-673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madhi SA, Klugman KP Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haynes LN. Progress and challenges in RSV prophylaxis and vaccine development. J Infect Dis. 2013;208:S177–S183. doi: 10.1093/infdis/jit512. [DOI] [PubMed] [Google Scholar]
- 41.The National Institute for Communicable Diseases (NICD) of the National Health Laboratory Service (NHLS) Healthcare Workers Handbook of Influenza. Johannesburg, South Africa: National Institute for Communicable Diseases; 2012. [Google Scholar]
- 42.Madhi SA, Dittmer S, Kuwanda L, et al. Efficacy and immunogenicity of influenza vaccine in HIV-infected children: a randomized, double-blind, placebo controlled trial. AIDS. 2013;27:369–379. doi: 10.1097/QAD.0b013e32835ab5b2. [DOI] [PubMed] [Google Scholar]


