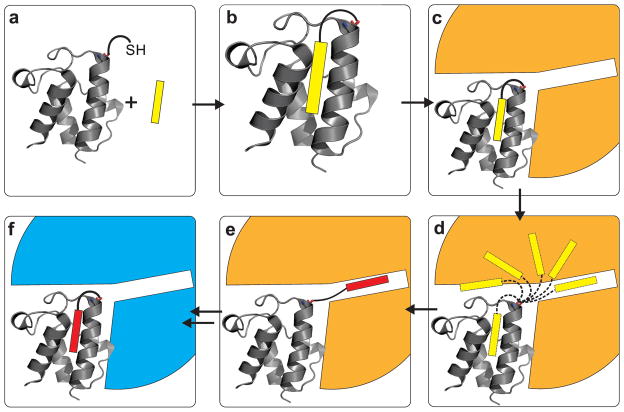
Figure 2.
Cartoon of cargo sequestration and chain-flipping mechanism by acyl carrier proteins. a) the acyl carrier protein (ACP, grey protein cartoon structure) is post-translationally modified with a 4′-phosphopantetheine arm, bearing a terminal thiol. b) when a fatty acid intermediate (yellow rectangle) is loaded onto the 4′-phosphopantetheine moiety, it is protected from the environment by sequestration in the inner core of the ACP. c) a partner protein (orange) binds to the ACP. d) the protein-protein binding event induces a conformational change in the carrier protein, allowing the cargo to flip out of the inner core into the active site of the partner enzyme (the “chain-flipping mechanism”). e) chemical transformation of the fatty acid intermediate (yellow > red) occurs in the active site of the partner enzyme. f) after the reaction is complete the partner enzyme leaves, and another partner protein (blue) can bind to the ACP.