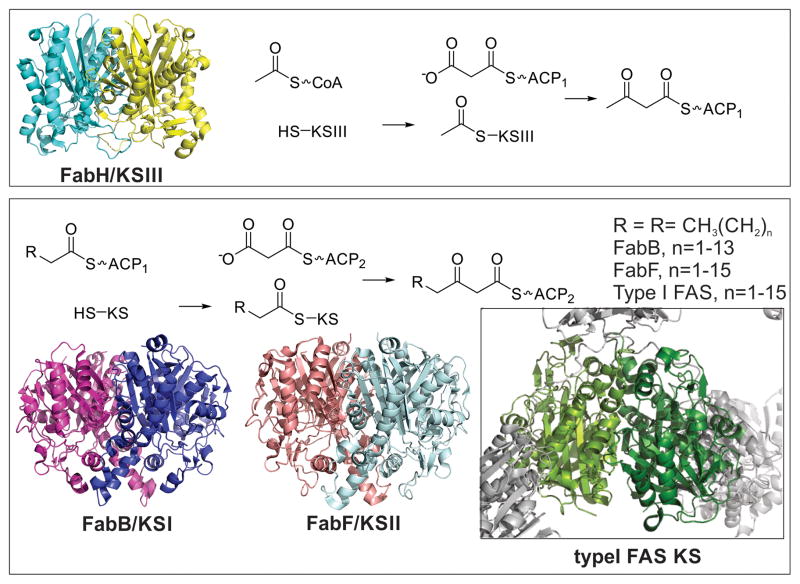
Figure 5.
Ketoacyl synthases. The ketoacyl synthase dimer is highly conserved throughout all branches of life. Top: FabH (KSIII, PDB: 1EBL) and FabD (MCAT) are responsible for initiating fatty acid biosynthesis. The acetyl group of acetyl-CoA is transferred to the active site cysteine residue of KSIII. Malonyl-ACP (the product of FabD/MCAT) binds to KSIII and a new carbon-carbon bond is formed, while CO2 is released. Bottom: FabB (KSI, PDB: 1G5X) and FabF (KSII, PDB: 2GFW) extend the chain further by first transfer of a fatty acid from an alkyl-ACP species to a conserved cysteine residue on the ketoacyl synthase. A different carrier protein loaded with a malonyl moiety binds to the loaded KS and a new carbon-bond is formed, while CO2 is released. The type I FAS KS from pig is shown as insert, showcasing the highly conserved fold and dimer structure of these enzymes.