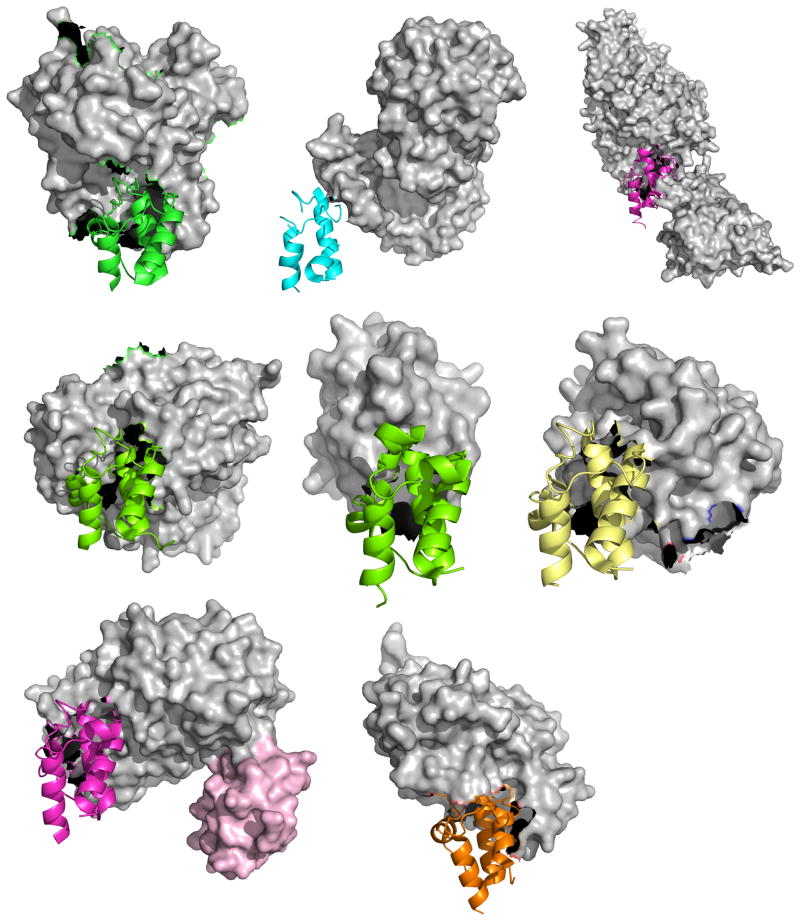
Figure 8.
Comparison of all eight ACP-partner protein X-ray crystal structures deposted in the PDB, from the perspective of the ACP, showing the similar binding orientation of the ACP, but the different binding motifs of the partner proteins. From top to bottom and left to right: PDB: 1F80, E. coli PPTase AcpS with E. coli holo-AcpP, PDB: 2FHS, E. coli FabI with E. coli AcpP, PDB: 2XZ0, Ricinus communis stearoyl desaturase with R. communis ACP bearing a phosphoserine, PDB: 3EJB, B. subtilis P450BioI with E. coli AcpP bearing tetradecanoic acid, PDB: 3NY7, STAS domain of E. coli YchM with E. coli AcpP bearing a terminal acid propionic acid thioester, PDB: 4ETW, E. coli BioH with E. coli pimeloyl-AcpP, PDB: 4KEH, E. coli FabA mechanistically crosslinked with E. coli AcpP bearing a non-hydrolyzable pantetheinamide crosslinker (in light pink surface the second ACP) and PDB: 2CG5, Homo sapiens AASDHPPT with human FAS apo-ACP excised from the human type I FAS.