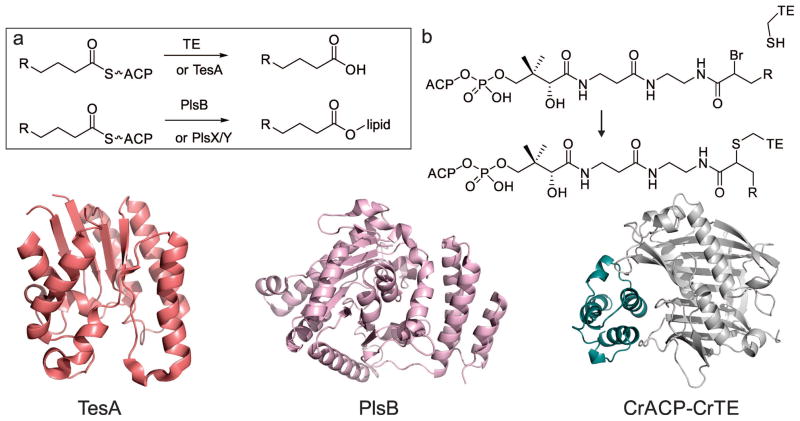
Figure 9.
Fatty acid biosynthesis termination by thioesterases or acyltransferases. Top: a) In plants, green algae and some bacteria, dedicated hotdog-fold acyl-ACP thioesterases (e.g. CrTE, Chlamydomonas reinhardtii Fat1) are responsible for hydrolyzing fatty acids, with a certain chain-length off the ACP (e.g. CrACP, C. reinhardtii ACP). TesA is a multifunctional enzyme that can hydrolyze fatty acids off the ACP, but this is presumably not its primary function in E. coli. In bacteria, dedicated acyl-transferases, like PlsB, trans-esterify lipid headgroups with a fatty acid, directly from the ACP. Alternatively, E. coli uses PlsX to synthesize phospho-fatty acids, which are loaded onto lipids using PlsY. b) Mechanistic crosslinking of ACP to TE using α-bromo acid pantetheinamide probe. Bottom: structures of TesA (PDB: 1IVN), PlsB (PDB: 1K30) and a docked structure of chloroplastic C. reinhardtii ACP (model) with C. reinhardtii TE FAT1 (model).