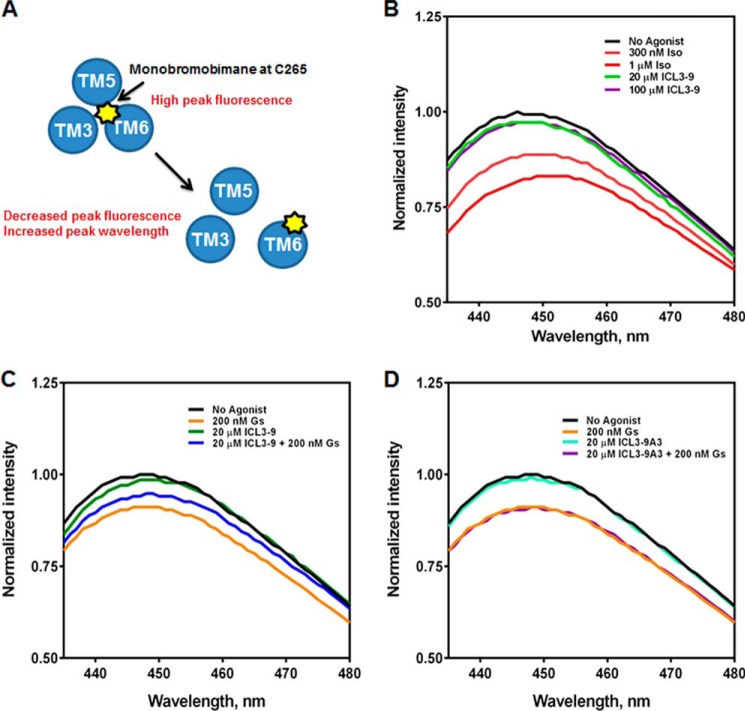
FIGURE 10.
ICL3-9 promotes unique conformational changes in the β2AR that promote Gs coupling. A, monobromobimane is an environmentally sensitive fluorophore that when chemically conjugated to β2AR-Cys265 can indicate local conformational changes. When the β2AR is in an inactive state, Cys265-monobromobimane is occupying a hydrophobic pocket and fluorescence is high. Upon receptor activation, a large outward movement of TM6 repositions Cys265 to be solvent-exposed resulting in decreased fluorescence and an increase in λmax. B, lipid bicelles containing 50 nm monobromobimane-labeled β2AR were incubated for 10 min at 25 °C with isoproterenol (300 nm or 1 μm) or ICL3-9 (20 or 100 μm) in 20 mm HEPES, pH 7.5, 100 mm NaCl. Fluorescence spectra were gathered by excitation at 370 nm and scanning 430–490 nm at 1.0 nm/s. 0.5% DMSO was included in non-pepducin-stimulated samples. C, lipid bicelles containing 50 nm monobromobimane-labeled β2AR were incubated for 10 min at 25 °C with 20 μm ICL3-9. 200 nm Gs was then incubated for 20 min at 25 °C in co-treatment studies. 0.1% DMSO was included in non-pepducin-stimulated samples. D, lipid bicelles containing 50 nm monobromobimane-labeled β2AR were incubated for 10 min at 25 °C with 20 μm ICL3-9A3. 200 nm Gs was then incubated for 20 min at 25 °C in co-treatment studies. 0.1% DMSO was included in non-pepducin-stimulated samples.