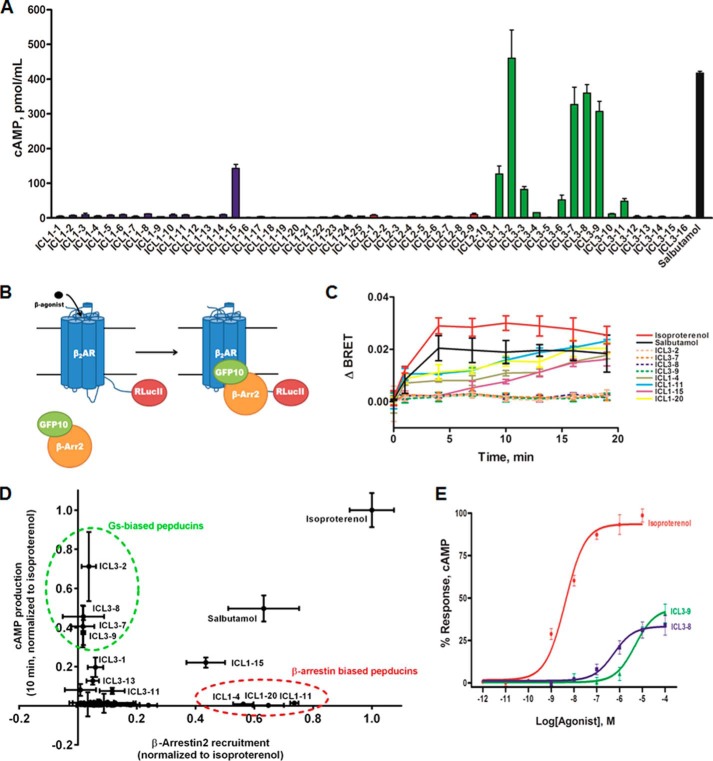
FIGURE 2.
Analysis of β2AR pepducins for cAMP production and β-arrestin binding. A, cAMP assay was performed in HEK 293 cells. Cells were stimulated with 10 μm pepducin or 5 μm salbutamol in DMEM with 10% FBS in the presence of 500 μm IBMX. cAMP was measured at 10 min by ELISA. Data are represented by the mean of three independent experiments ± S.D. B, schematic of β-arrestin recruitment analysis by BRET. Upon β-agonist stimulation, GFP10-β-arrestin2 is recruited to β2AR-RLucII. Upon β-arrestin2 binding to the β2AR, GFP10 will be within the BRET radius of RLucII allowing GFP10 emission readout to be indicative of β-arrestin recruitment. C, HEK 293 cells co-transfected with GFP10-β-arrestin2 and β2AR-RLucII were preincubated with coelenterazine 400a for 2 min and stimulated with 10 μm pepducin, 1 μm isoproterenol, or 5 μm salbutamol for the indicated times. BRET was monitored at the indicated times post-addition. Data are expressed as ΔBRET as the background BRET has been subtracted. The data are represented by the means of four independent experiments ± S.D. D, cAMP output (10 min) as a function of β-arrestin recruitment reveals multiple Gs-biased and β-arrestin-biased pepducins. Balanced agonists, such as isoproterenol, can effectively promote both cAMP production and β-arrestin recruitment with similar efficacies. An agonist that promotes cAMP production more effectively than β-arrestin recruitment is a Gs-biased agonist (i.e. ICL3-8 and ICL3-9), whereas agonists that couple β-arrestins more effectively than stimulating cAMP production are considered β-arrestin-biased. E, HEK 293 cells were stimulated with various concentrations of isoproterenol, ICL3-8, or ICL3-9 for 10 min in the presence of 500 μm IBMX. ICL3-8 has an EC50 of 577 ± 14 nm, whereas ICL3-9 has an EC50 of 4.7 ± 0.1 μm. cAMP production is represented as percentage normalized to maximal isoproterenol stimulation. The data are represented by the mean of three independent experiments ± S.D.