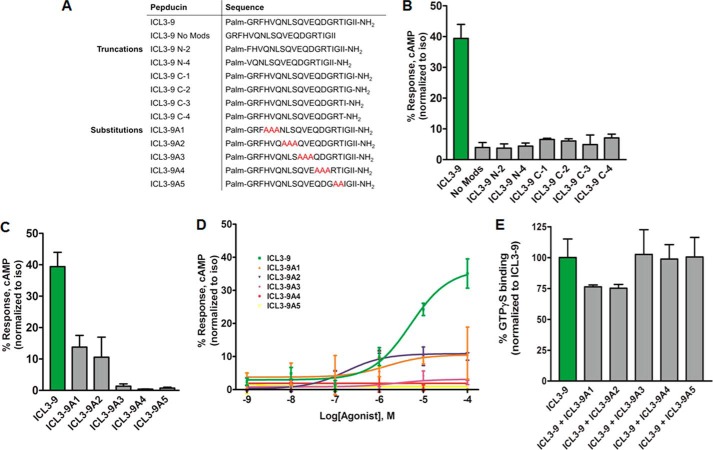
FIGURE 7.
ICL3-9 truncations and mutations modulate pepducin efficacy and potency. A, sequences of ICL3-9 truncation and substitution variants. B, HEK 293 cells were stimulated with 10 μm ICL3-9 truncation variants in DMEM with 10% FBS in the presence of 500 μm IBMX. 0.05% DMSO was added to cells not stimulated by pepducin. cAMP was measured at 10 min by radioimmunoassay. Data are represented by the mean of three independent experiments ± S.D. C, HEK 293 cells were stimulated with 10 μm ICL3-9 mutations in DMEM with 10% FBS in the presence of 500 μm IBMX. 0.05% DMSO was added to cells not stimulated by pepducin. cAMP was measured at 10 min by radioimmunoassay. Data are represented by the mean of three independent experiments ± S.D. D, cAMP was measured in HEK 293 cells stimulated with ICL3-9 substitution variants for 10 min in the presence of 500 μm IBMX. 0.05% DMSO was added to cells not stimulated by pepducin. cAMP production is normalized to isoproterenol stimulation. The EC50 of ICL3-9A1 is ∼1.5 ± 0.8 μm and the EC50 of ICL3-9A2 is ∼0.18 ± 0.24 μm. E, lipid bicelles containing reconstituted 12.6 nm β2AR/180 nm Gs or 180 nm Gs alone were preincubated with 5 μm of the ICL3-9 substitution variants for 10 min at room temperature and stimulated with 5 μm ICL3-9 in the presence of 38.5 nm [35S]GTPγS in assay buffer (20 mm HEPES, 7.5, 150 mm NaCl, 1 mm MgCl2). Samples were isolated by rapid filtration on BA85 filters. The data are represented by the mean of three independent experiments ± S.D.