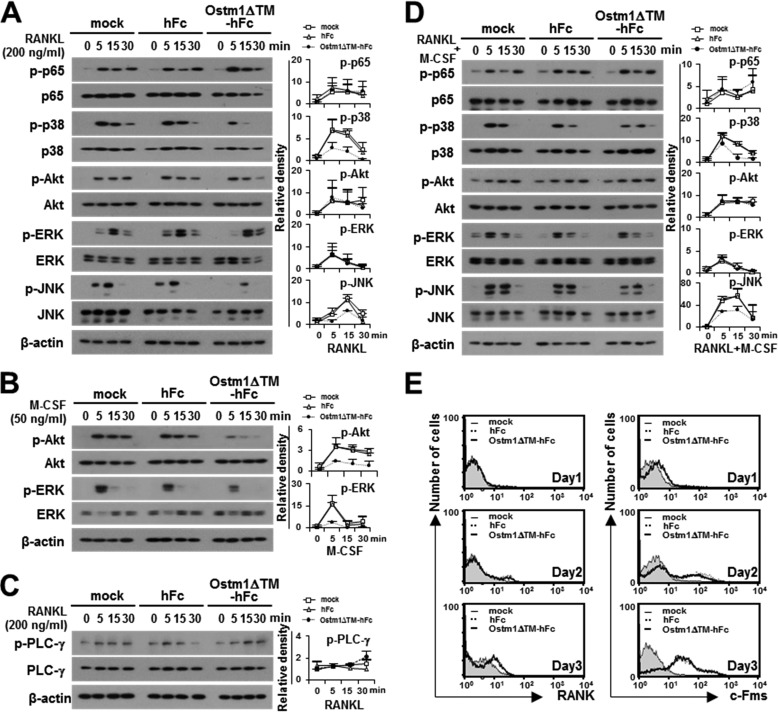
FIGURE 4.
The effect of the truncated OSTM1 on the signaling pathways involved in OC differentiation. A, effect of OSTM1ΔTM-hFc treatment on RANKL-induced signaling. BMMs pretreated with purified proteins (25 μg/ml) for 2 h. The pretreated BMMs were further stimulated with RANKL for the indicated times. The cells were then analyzed through immunoblotting with antibodies recognizing phosphorylated and total p65, p38, Akt, ERK, and JNK. β-Actin was used as the loading control. The relative level of phosphorylated forms was calculated after normalization to total protein input (right). B, effect of OSTM1ΔTM-hFc treatment on M-CSF-induced signaling. The M-CSF-induced signals were analyzed through immunoblotting with antibodies recognizing phosphorylated and total Akt and ERK. The relative level of the phosphorylated form was calculated after normalization to total protein input (right). C, effect of OSTM1ΔTM-hFc treatment on RANKL-induced co-stimulatory signaling. The RANKL-induced co-stimulatory signals were analyzed through immunoblotting with antibodies recognizing phosphorylated and total PLC-γ. The relative level of the phosphorylated form was calculated after normalization to total protein input (right). D, effect of OSTM1ΔTM-hFc treatment on the stimulation of both RANKL and M-CSF. The relative level of the phosphorylated form was calculated after normalization to total protein input (right). E, expression of RANK and c-Fms on the surface of OCs in response to OSTM1ΔTM-hFc treatment. BM cells were differentiated into BMOCs using M-CSF (50 ng/ml) and RANKL (200 ng/ml) for 1–3 days. The cells were stained with antibodies against RANK (left panels) or c-Fms (right panels). Gray with thin solid lines, mock; dotted lines, hFc; thick solid lines, OSTM1ΔTM-hFc. Error bars, S.D.